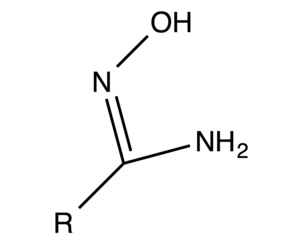
 |
| Fig. 1: An amidoxime functional group. |
With energy demand expected to increase due to the rise of developing nations and with increased concern over fossil fuel depletion, nuclear energy is becoming an attractive option to meet rising energy demand, especially since there are no CO2 emissions associated with the operation of a nuclear power plant. Terrestrial uranium reserves, while plentiful, are not infinite. As discussed previously by Chan and Ferguson, an alternative supply of uranium can be found in the oceans. [1-3] Concentrations of uranium in seawater are extremely low (about 3.3 ppb), but given that the volume of the ocean is about 1.4 billion cubic kilometers, there are an estimated 4.5 billion tons of uranium in the oceans, a thousand times more uranium than in terrestrial ores. [3] It has been estimated that seawater uranium reserves contain 350 YJ (1024 J) of available energy, compared to 1 YJ of available energy in terrestrial uranium. [4] Recovery of seawater uranium also has none of the environmental problems associated with mining of depleted ores and thus could be an attractive alternative to traditional uranium reserves in the future. [5]
Uranium exists in the oceans as part of the uranyl tricarbonate anion [UO2(CO3) 3]4-. [6] Efforts to extract this form of uranium from seawater began in the 1960s. While most of the research has come from Japan, there has also been noteworthy work from India and Germany. Early research efforts determined that adsorption was the most effective method to extract uranium from seawater rather than other chemical separations techniques such as solvent extraction or ion exchange. [3] Initially, hydrous titanium dioxide (TiO2) was considered a promising adsorbent, so much so that in Japan from 1981-1988, the Agency for Natural Resources and Energy, the Ministry of International Trade and Industry, and the Metal Mining Agency of Japan teamed up to operate an experimental marine uranium adsorption plant based on TiO2 adsorbents. Results from the plant showed that the adsorption capability of the TiO2 was only 0.1 g U/kg-adsorbent, which was much too low for practical purposes. Furthermore, plant operation showed that the TiO2 adsorbent degraded significantly over time and was not mechanically strong enough to withstand operation in ocean water. [3]
Once it had been shown that TiO2 was not a viable solution to seawater uranium extraction, research in Japan turned to finding a better uranium adsorbent. Eventually, it was found that amidoxime functional groups were more effective and more durable than hydrous titanium. To produce adsorbents with this material, polyethylene was irradiated with an electron beam and grafted with acrylonitrile. One of the functional groups on the polymer chain was then replaced with an amidoxime group, resulting in a polymer-backbone material with the correct materials chemistry to adsorb uranium. [3]
With the development of the amidoxime material came interest in pilot plants, not only in Japan, but also in India and Germany. In Japan, amidoxime-based fibers in the form of both stacks and braids were tested in marine environments. The fiber stacks were tested from 1999 to 2001 in the Pacific Ocean off the coast of the Aomori prefecture of Japan and showed an average uranium adsorption capability over 30 days of 0.5 g U/kg-adsorbent, five times higher than that of the TiO2. The braided fibers, which mimicked the vertical orientation of kelp or seaweed when anchored to the ocean floor, were tested in the Pacific Ocean near the Okinawa region of Japan and showed an average uranium uptake of 1.5 g U/kg-adsorbent. This was likely due to better surface contact between the braided fibers and the ocean water. [3]
Outside of Japan, researchers in India and Germany have also developed pilot plants for seawater uranium extraction. In India, a collaboration between the Bhabha Atomic Research Centre in Mumbai and the Commissariat à l'Energie Atomique in France led to a pilot plant that took in concentrated brine from a desalination plant. The materials used here were amidoximated macroporous membranes, which are slightly different than the Japanese materials. From the experiments, the team found that their materials had an average uranium uptake of .060-.160 g U/kg-adsorbent after 12 to 24 days of soaking. The lower adsorption levels are likely due to the fact that the adsorbents also collected vanadium. In Germany, researchers at the Jülich Nuclear Research Centre developed a cross-linked poly(acrylamidoxime) material and tested it in the German North Sea and in the Gulf Stream near Miami, Florida. They found that their materials had uranium loadings ranging from 100-3000 ppm and had good chemical and mechanical stability in ocean water. [3]
Research efforts from Oak Ridge National Laboratory in collaboration with the University of Tennesse have explored advanced polymer support materials for amidoxime synthesis. [5-8] The research group started first by testing mesoporous carbon substrates grafted with polyacrylonitrile in simulated seawater (with only a fraction of the heavy metals found in actual seawater) but moved on to testing mesoprous carbon substrates grafted with a copolymer in real, filtered seawater. [4,6] From the latter tests, they found that their best candidate material had a uranium adsorption capacity of 0.55 g U/kg-adsorbent when shaken for 28 days in seawater. Following their work with carbon substrates, they continued on to copolymer substrates in order to reduce the mass of the adsorbent and thereby improve the uranium uptake capacity ratio. [5,7] The group developed a nanoporous polymer that used a vinylbenzyl chloride monomer backbone with a divinylbenzene cross-linking agent. This material gave the most promising results yet, showing a uranium uptake capacity of 1.99 g U/kg-adsorbent after being shaken for 27 days in seawater. Further work needs to be done to determine the material's performance after many use cycles and to determine its mechanical and chemical durability in a marine setting.
Moving away from traditional amidoxime as the uranium-adsorbing agent, a group at the University of North Carolina Chapel Hill has started investigating metal-organic frameworks (MOFs) for seawater uranium extraction. [9] The researchers developed three different materials based on UiO MOFs (named UiO for the University of Oslo, where these frameworks were first developed) in which they replaced one of the amine groups with a phosphorylurea group. They tested their materials in water and simulated seawater. The most promising MOF had a uranium adsorption capacity of 188 g U/kg-adsorbent in simulated seawater, compared to a uranium adsorption capacity of 54 g U/kg-adsorbent for amidoxime resins in similar conditions. More work needs to be done to test the materials in actual seawater where there are competing heavy metals able to be adsorbed by the MOFs and to determine mechanical and chemical durability of the novel frameworks.
Tapping into resources and knowledge from the biological world, researchers at the University of Chicago and Peking University in collaboration with Argonne National Laboratory developed a novel protein for uranium binding. [7] Since no naturally-occurring proteins are known to bind uranium ions, the team ran computer simulations to find candidate biological binding groups and synthesize those into their own, new protein. Once they found the best candidate protein, they further improved it through a series of mutations to increase uranium binding affinity. The researchers then fixed the engineered protein onto both a sulfhydryl resin and E. coli cells and tested the material in synthetic seawater. In the former case, the protein bound uranium ions over 17 other competing metal ions with a selectivity factor of more than 10,000. In the latter case, the protein managed to extract over 60% of uranium ions from the simulated seawater. No work has been done with this new protein in actual seawater nor to assess its mechanical or chemical durability.
While there is still a lot of work that needs to be done with new materials for extraction of uranium from seawater, these materials have come a long way from the first efforts with hydrous titanium dioxide. New amidoxime-based materials, along with novel metal-organic frameworks and biological materials, have the potential to increase seawater uranium extraction efficiency to the point where it is an economically viable alternative to mining uranium ore. Before this can happen, however, further research is needed to test these materials in realistic marine settings and pilot plants.
© Carol Regalbuto. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] B. Chan, "Amidoxime Uranium Extraction From Seawater," Physics 241, Stanford University, Winter 2011.
[2] K. Ferguson, "Uranium Extraction From Seawater," Physics 241, Stanford University, Winter 2012.
[3] L. Rao, "Recent International R&D Activities in the Extraction of Uranium from Seawater." Lawrence Berkeley National Laboratory, LBNL-4034E, May 2011.
[4] W. A. Hermann, "Quantifying Global Exergy Resources," Energy 31 1685 (2006).
[5] Y. Yue et al., "Seawater Uranium Sorbents: Preparation from a Mesoporous Copolymer Initiator by Atom-Transfer Radical Polymerization," Angew. Chem. Int. Ed. 52 13458 (2013).
[6] J. Gorka et al., "Sonochemical Functionalization of Mesoporous Carbon for Uranium Extraction from Seawater," J. Mater. Chem. A 1 3016 (2013).
[7] Y. Lu, "Uranium Extraction: Coordination Chemistry in the Ocean," Nat. Chem. 6 175 (2014).
[8] Y. Yue et al., "Polymer-Coated Nanoporous Carbons for Trace Seawater Uranium Adsorption," Sci. China Chem. 56, 1510 (2013).
[9] M. Carboni et al., "Highly Porous and Stable Metal-Organic Frameworks for Uranium Extraction," Chem. Sci. 4 2396 (2013).