 |
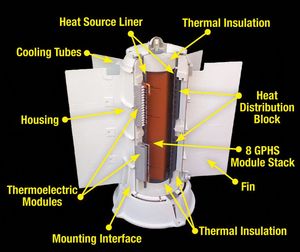
| Fig. 1: Schematic of a typical radioisotope thermoelectric generator. (Courtesy of NASA.) |
Nuclear processes have long been exploited for generating heat and electricity for energy needs. In most of these cases, both the methods of generation and eventual applications are often associated with larger-scale structures (power plants) and distribution (national usage). However, there are indeed much smaller scale situations involving the production of energy using nuclear processes. One of these examples is the use of radioisotope thermoelectric generators (RTGs). RTGs are devices that convert the waste heat given off by radioactive decay processes into useable electrical energy and are often installed in space-bound objects that require energy and other remote structures/machines that cannot obtain energy efficiently by any other means. These include satellites, probes, and remote lighthouses. Ideally, RTGs are established in systems under some of the following circumstances:
Unable to be continually maintained and serviced
Incapable of generating solar energy efficiently
Need to remain operating without human aid for long durations of time
Minimal human interaction
Based on these circumstances, the chief usage of RTGs is in fully automated systems that will not experience human contact for periods of time longer than other sources of energy, such as batteries and fuel cells, can sustain and in environmental conditions that are not conducive to generating energy by natural means (solar, wind, etc.). The following provides an overview of radioisotope thermoelectric generators including descriptions of their designs and how they operate, some examples of modern applications, and a few comments on their general safety.
The typical design of an RTG is actually relatively simple and straightforward, consisting of two crucial ingredients: fuel that will decay radioactively and a large set of thermocouples to convert heat into electricity. Figure 1 is a cutaway view of an archetypal modern RTG, showing all of the interior parts. The fuel is located behind the thermal insulation layer and thermocouples are lined in modules throughout the sides of the RTG. Specifically, this image shows a Multi-Mission Radioisotope Thermoelectric Generator (MMRTG), which will be discussed again later.
The selection of fuels for RTGs is certainly not a trivial matter; there are several criteria that isotopes must pass in order to stand as candidates. In fact, the initial research carried out by Dr. Bertram Blanke on the development of RTGs evaluated over 1300 radioactive isotopes for the project, but only found that 47 of them had the suitable characteristics. [1] These characteristics include:
Ability to produce high energy radiation
Tendency to produce radiation decay heat
Possession of long half-life for continuous energy production
Large heat power-to-mass (or density) ratio
The first factor is rather obvious and simply a statement of the fact that whatever isotope is chosen as a fuel must be able to release enough energy in its decay process to serve as a practical and fruitful enough source for thermoelectric conversion. This characteristic alone does not exclude many isotopes, but the next trait of being able to produce radiation decay heat is a stricter guideline.
The heat associated with the majority of radioactive decays occurs as a result of the decay products being absorbed into various materials and causing thermal atomic motion. For a compact device such as an RTG, effective generation of heat must occur on a relatively short length scale, within the confines of the device walls. This directly equates to the need for decay products that have short absorption lengths. Looking over the various types of radioactive decay (alpha, beta, gamma), the order of radiation absorption lengths from shortest to longest are alpha, beta, and then gamma. This means that over a finite length of material contained within an RTG, the most heat will be produced by alpha decay. Therefore, in choosing an appropriate RTG fuel, it is best to find isotopes that decay with alpha radiation first. It should be noted, however, that isotopes yielding beta and gamma radiation can be viable candidates too as long as the proper materials are used for absorption and conversion into heat for these types of radiation.
To continue, the next criterion for choosing a fuel is a long half-life. Considering that most RTGs will end up in isolated environments with very little human presence and thus chances to re- fuel, the need for an isotope that can continuously produce energy for long periods of time is rather obvious. Of course the exact isotope half-life requirements will vary based on the situation, but generally, longer half-lives are desirable, leading to sustained levels of energy production. The final parameter for choosing an acceptable isotope is mostly a statement of size efficiency. To create a compact RTG device, every element must be reasonably small including the fuel content. Even if a particular isotope passes all of the above criterion for fuel choice, if it takes an inordinate amount of the substance to produce the required energy, it will be less attractive. For RTGs that will end up in small extraterrestrial vehicles/applications, weight and efficiency end up being the most important factors.
Based on all of the above factors, the most frequently used isotopes for RTG fuels include Plutonium-238 (Pu-238), Strontium-90 (Sr-90), and Curium-244 (Cm-244) with Pu-238 being the most cited fuel on most resources about RTGs. In fact, frequent use of Pu-238 for RTGs, including its employment in almost two dozen space missions, has led to a recent shortage of the heavily depended- upon material. [2] Pu-238 satisfies all of the above RTG fuel requirements with high radiation output, primarily alpha decay channels and thus low shielding needs, a very long half-life of 88 years, and a fuel pellet packaged into the size of a marshmallow as seen in Fig. 2. Other isotopes can also serve as fuels, but suffer from various disadvantages in comparison with Pu-238 including more shielding requirements due to non-alpha radiation decay, shorter half-lives, and generally less radiation output.
 |
| Fig. 2: An image of a marshmallow-sized Pu-238 pellet. (Source: Wikimedia Commons.) |
With the RTG fuel criteria considered, a discussion of the other crucial ingredient of RTGs, the thermocouples, is needed. Once an isotope fuel pellet is installed in an RTG, it begins to decay radioactively, creating heat that is collected by heat distribution blocks. These blocks then send the heat to sets of thermocouples that convert the heat into useful electricity. Thermocouples have long been in use and are not particularly complex or nascent. They rely on a single, simple principle called the Seebeck effect, first discovered by Thomas Seebeck in 1821, which observes that a differential in temperature between two ends will lead to an electric voltage and vice-versa. Thus, if a device can be constructed to achieve a strong temperature gradient in an electrically conductive element, then a voltage difference can be induced along with a useful electrical current. This typically requires the use of materials with low thermal conductivity, which would allow for a wide temperature difference to accumulate between two ends, and high electrical conductivity so that currents can easily flow. Currently, thermocouples used in RTGs contain high-performance thermoelectric materials such as bismuth telluride (BiTe), lead telluride (PbTe), tellurides containing antimony, germanium, and silver (TAGS), and silicon germanium (SiGe). [3] These materials absorb the heat generated by the RTG isotope fuel, create a dramatic temperature gradient due to their low thermal conductivities, and then produce electrical currents that are outputted by the RTG to elements that need to be powered.
Despite the straightforward implementation of thermocouples in RTGs, the chief disadvantage of using them is their low conversion efficiency of heat to electrical energy. Conversion rates for the materials listed above typically lie in the range of between 5 to 9%. [4] Although other options exist, very few match the low cost, low weight, and ease-of-use of thermocouples.
The simple design of RTGs leads to their utilization in many applications fitting the parameters listed in the introduction, both on Earth and in space. On Earth, RTGs have been used in unmanned facilities such as hundreds of old, abandoned Russian lighthouses and various U.S.- commissioned arctic monitoring sites. [5,6] The keys to these terrestrial uses are that the RTGs have been placed in remote areas not frequently accessed by humans for maintenance and used in facilities that will remain at their locations for extended periods of time, lasting decades. This justifies the use of these potentially hazardous nuclear-powered RTGs on Earth, minimizing danger to human beings. The safety element of RTGs will be discussed briefly in the next section.
The most impactful usage of RTGs has been in a variety of interstellar projects including a fairly large variety of space probes sent to the Moon, flights to the outer planets of the Solar System such as Pioneer and Voyager, and most recently, the robotic rover Curiosity sent to Mars. [7-9] The implementation of an RTG into the Mars rover is particularly interesting here since it provides an opportunity to discuss the most modern iteration of space-bound RTGs, named multi- mission radioisotope thermoelectric generators (MMRTGs).
Functionally, MMRTGs actually retain exactly the same ingredients as all other RTGs described above, even using Pu-238 as their source of radiation fuel. [9] This is a testament to the true reliability and effectiveness of the original RTG idea and model. Perhaps the only major upgrade is the use of newer and improved thermoelectric converters, namely PbTe / TAGS devices designed to squeeze out between 100 and 125 Watts of electrical energy from Pu-238 fuel pellets over the course of 14 years. [10,11] This MMRTG design has been reliably running Curiosity since its landing on August 6, 2012 and will most likely be used in future space-bound probes and modules.
As with the implementation of any nuclear-based processes into functioning devices, there is always concern over human safety and radioactive contamination. Even though RTGs are designed to function in remote environments with sparse human populations, the worries are not totally unwarranted as there are plenty of questions regarding the event of RTG fuel leaks or possible explosions while launching space-bound RTGs. In the worst-case scenarios of these situations, there would be substantial radioactive contamination in the environment along with the potential for radiation damage to humans. This makes the use and launching of RTGs at least semi-controversial. However, in practice, there are safety measures applied to minimize the risks of radioactive contamination from RTGs. For instance, in the NASA mission to Saturn featuring the Cassini-Huygens probe, the RTG isotope fuel was stored in high-strength blocks of graphite and surrounded by a layer of iridium metal in order to curb the risk of accidental explosions. [12] These graphite blocks have proven to be successful in preventing radiation contamination as in the case of the famed failed Apollo 13 landing in 1970, which left its RTG in the ocean after its return to Earth, but with no detectable plutonium contamination. [13] In the end, despite potential radiation risks, the advantages of RTG use far outweigh all other factors.
The use of RTGs is a perfect example of the application of nuclear processes on smaller size scales. They are widely implemented in space-bound projects that require energy where resources for power are meager along with terrestrial projects in areas with very little human presence. RTG use will only increase in the future as they are effective sources of energy for specific situations, although different fuel sources must be discovered and effectively integrated with the gradual depletion of Pu-238.
© Mason Jiang. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] B. C. Blanke et al., "Nuclear Battery Thermocouple-Type Summary Report," Monsanto Research Corporation, MLM-1127, 15 Jan 62.
[2] D. Kramer, "Shortage of Plutonium-238 Jeopardizes NASA's Planetary Science Missions," Physics Today 64, No. 1, 24 (2011).
[3] G. R. Schmidt, T. J. Sutliff, and L. A. Dudzinksi, "Radioisotope Power: A Key Technology for Deep Space Exploration," in Radioisotopes - Applications in Physical Science, ed. by N. Singh (InTech, 2011), p. 419.
[4] Energy-Efficient Technologies for the Dismounted Soldier (National Academy Press, 1997), p. 218.
[5] M. K. Sneve, "Remote Control," Int. Atomic Energy Agency Bull. 48, No. 1 , 42 (2006).
[6] "Power Sources for Remote Arctic Applications," U.S. Office of Technology Assessment, OTA-BP-ETI 129, June 1994.
[7] D. Harland, Apollo 12 - On the Ocean of Storms (Springer, 2010), p. 269.
[8] G.L. Bennett, "Space Nuclear Power: Opening the Final Frontier", Am. Ins. Aero. Astro., AIAA 2006-4191, June 2006.
[9] W.J. Hennigan, "Mars Rover Draws on Nuclear Power for Trek Around Red Planet," Los Angeles Times, 5 Aug 12.
[10] F. Ritz and C. E. Peterson, "Multi-Mission Radioisotope Thermoelectric Generator (MMRTG) Program Overview," Proc. 2004 IEEE Aerospace Conf (IEEE, 2004).
[11] A. K. Misra, "Overview of NASA Program on Development of Radioisotope Power Systems with High Specific Power," Am. Inst. Aero. Astro., AIEE 2006-4187, June 2006.
[12] J.A. Rumerman, "NASA Historical Data Book Volume VII U.S. National Aeronautics and Space Administration NASA SP-2009-4012, 2009, p. 741.
[13] W. J. Broad, "Saturn Mission's Use of Plutonium Fuel Provokes Warnings of Danger," New York Times, 8 Sep 97.