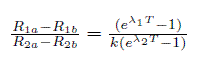
The majority of ionizing radiation around us (and in us) comes from natural sources: food, rock, soil, water, animals, plants, gas in the atmosphere. Like stable elements, radioactive isotopes help define the structure of materials and the way in which they interact with their environment. We can use their physical signatures, like half lives and relative concentrations, to determine the age and origin of composite materials -- minerals, animal bones, planets, etc.. Familiar to geoscientists, some of these techniques are Uranium/Lead(Lead/Lead) Dating, Potassium/Argon Dating, and Carbon Dating.
We know of three hundred and thirty nine naturally occurring isotopes. [1] The majority of them are theoretically unstable, but decay slowly enough that their decay processes are undetectable. A primordial isotope is one that has existed in it's present state on Earth for the lifetime of the earth. Of these, the decay processes of Uranium and Thorium contribute the most to the background radiation spectrum (by a substantial margin). [2]
Three isotopes dominate the background ionizing radiation spectrum. Potassium-40 has the highest concentrations because it is ubiquitous in biological materials, while Uranium and Thorium are found primarily in rocks and soil (particularly igneous rocks like granite [3]). In fact, most of the radiation to which we are exposed comes from the accumulated Potassium in our bodies and concentrations of these three isotopes in the soil.
In 1950, E.C. Anderson and W.H. Langham conducted an experiment that showed that the amount of potassium in the body spiked around age 10 for women and age 20 for men. This data indicates that the average concentration for both sexes over all ages is approximately 2.0 g/kg. [4]. Potassium emits about 31.2 beta rays per gram per second and 3.6 gamma rays per gram per second. [5] Therefore a person weighing 180 lbs (82 kg) is exposed to 5117 beta rays per second and 590 gamma rays per second from the potassium in their bodies. Because soil is a major source of gamma radiation, there has been an international effort to survey the gamma ray spectra of soil in various parts of the world. The UN Scientific Committee released a report in 2000 which gives the average activity of Uranium, Thorium, and Potassium in Soil samples. These are 40 Bq/kg, 40 Bq/kg, and 370 Bq/kg respectively. [6] If we want to consider the amount of radiation from the soil to which we're exposed, we have to first halve the total activity because half of the radiation from a flat layer of soil radiates downward. This gives us 225 Bq/kg. Next, we assume that only radiation from soil within an area of 1 square ft of our feet reaches us. If we only consider the first half-foot of soil this corresponds to about 50 lbs(25kg). Therefore, we are exposed to about 5625 Bq or 5625 gamma rays per second from average soil sources. From our bodies and the soil at our feet alone (excluding cosmic rays which contribute significantly less) the average background level consists of about 6215 gamma rays per second and 5117 beta rays per second. To put this in perspective, 1 curie is the number of decays per second of on gram of radium and is equal to 37 billion. This stuff was played with daily by the early explorers of nuclear energy.
Ernest Rutherford first suggested using radioactivity for geological dating in 1904. [7] However, it took another half century for researchers to discover isotopes, and then realize that Uranium and it's decay product lead could be used to accomplish the feat. [7] In January 1956, Claire Patterson published a paper that utilized different lead isotope ratios to determine the age of meteorites and the earth (Uranium/Lead Dating). The latter measurement turned out to be very accurate and is the method by which present calculations are made. This dating process is accomplished by plotting the ratios of lead isotopes in meteorites from different sources and utilizing the measured decay rates to determine the necessary, self consistent time of creation (see equation below).
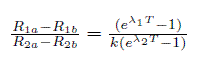
Here, R1 = Pb207/Pb204 and R2 = Pb206/Pb204 for two materials(meteorites) a and b. &lambda1 is the uranium(235) decay constant -- 9.72x10-10 yr-1, and &lambda2 is the uranium(238) constant -- 1.54 x 10-10 yr-1. K is the ratio of uranium(238) to uranium(235) which was about 137.8 when Patterson first published his results. [8] The object of this dating technique is to find a sample that accurately represents the age of system, and test whether the lead/lead and lead/uranium ratios fit the meteorite data. If it does, then the sample age can be deduced.
Another common technique used to date materials is potassium-argon dating. Because potassium is ubiquitous in nature, this type of dating is very useful. It was first accomplished by analyzing samples with significant concentrations of potassium that could be aged using uranium/lead dating. [8] It assumes a decay process of K40 -> Ar40. Two constants govern the decay process: &lambda and R. &lambda is the total decay constant and R is the branching ratio (&lambdaK / &lambda&beta). &lambdaK is the decay constant for K40 -> Ar40 and &lambda&beta is the decay constant for K40 -> Ca40. [9] Using potassium-argon dating often poses measurement difficulties. This is due to large concentrations of atmospheric argon which must be differentiated from the Ar40 potassium decay product. The identification of atmospheric argon is possible because it contains a small (0.4%) amount of Ar38. However, this subtle difference can cause large errors in practice.
In 1957, J. R. Arnold and W. F. Libby published a paper that described a technique to count C14 isotopes in CO2. In this experiment, they irradiated a sample of graphite, burned the sample in oxygen, and were then able to measure an activity of 0.38 ± 0.01 microcuries per gram. This technique has been a enormous asset to life scientists interested in carbonaceous materials; it allows one to estimate the age of any organic substance with a large enough carbon mass. It is made possible through the irradiation of N14 in the upper atmosphere by cosmic rays. When nitrogen(14) isotopes absorb a neutron made available by high speed muons, they decay to C14 with half lives of about 5700 years. This is long enough for the carbon to enter the food chain via CO2. The isotope accumulates throughout most organisms' lives and decays after their deaths. With this information, the decay rate of a carbon sample is enough to estimate the sample's age.
The ability to utilize radioactive properties to determine the age and origins of terrestrial materials has greatly enhanced our understanding of the earth and beyond. This paper only highlights some of the early developments of these dating techniques. It by no means represents all of the technical nor theoretical advancements made in the last fifty years. Because radiation levels of common radioactive isotopes are so low (low enough that we did not know about them until discovered in higher concentrations), it is an exciting physical consequence that we can utilize these natural phenomena to extract information about our existence.
© Andrew Lange. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] "Interactive Chart of Nuclides," Brookhaven National Laboratory.
[2] G. Heusser, "Low-Radioactivity Background Techniques," Annu. Rev. Nucl. Part. Sci. 45, 543 (1995).
[3] M. Abusini, et al., "Determination of Uranium, Thorium and Potassium Activity Concentrations in Soil Cores in Araba Valley, Jordon," Radiation Protection Dosimetry 128, 213 (1995).
[4] E.C. Anderson and W.H. Langham, "Average Potassium Concentration of the Human Body as a Function of Age," Science 130, 713 (1959).
[5] W. R. Faust, "Specific Activity of Potassium," Phys. Rev. 78, 624(L) (1950).
[6] M. Charles, "UNSCEAR Report 2000: Sources and Effects of Ionizing Radiation," J. Radiol. Prot. 21, 83 (2001).
[7] G. B. Dalrymple, "The Age of the Earth in the Twentieth Century: a Problem (Mostly) Solved," Geol. Soc. London Special Publication 190, 205 (2001).
[8] C. Patterson, "Age of Meteorites and the Earth," Geochim. Cosmochim. Acta 10, 230 (1956).
[9] J. Lipson, "Potassium-Argon Dating of Sedimentary Rocks," Geol. Soc. Am. Bull. 69, 137 (1958).