 |
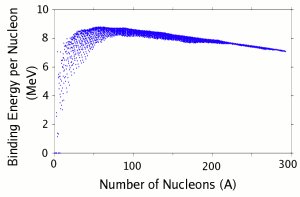
| Fig. 1: Plot of binding energy per nucleon vs. nucleon number for all known isotopes. Data from [4]. |
Recent focus on carbon dioxide emissions has led to nuclear energy once again being considered for electricity generation. [1-3] The previous science and innovation minister of Britain even moved to reclassify nuclear power as renewable, but is it really so outside of parliament? [4] Current commercial reactors use Uranium as fuel, and while Uranium has a fairly high average abundance of 2.7 parts per million in the Earth's crust, there are few economically viable sources. [5,6] Uranium is a fossil fuel without the carbon dioxide emissions, but the important question is how long it will last. As with oil, the lifetime of the Earth's Uranium supplies is controversial. [5,7] About 16% of the World's electricity is generated by nuclear reactors and if this percentage is to increase, the lifetime of Uranium supplies may be shorter than we think. [8]
The energy produced in conventional nuclear reactors comes from fission, where a heavy nuclei is split into two lighter nuclei. Nuclear fission releases energy because the smaller nuclei have a higher binding energy per nucleon than heavier nuclei, as can be seen on Figure 1. The peak in binding energy occurs at iron, which has 56 nucleons. Fission of one U-235 atom releases about 193.9 MeV of energy, which means that a coffee cup of Uranium (1 kg) can generate enough energy to power 2000 American homes for a year, assuming perfect conversion. [11, 12] Similarly, light nuclei like Hydrogen or Lithium can undergo fusion to release energy, but controlled fusion has not been realized yet. One might wonder where the enormous amount of energy contained in these heavy nuclei came from, and it is believed that they were generated in supernovae explosions. These extremely energetic star explosions give rise to the conditions needed to make heavy nuclei like Uranium and Thorium through exotic nuclear reactions. [11] The amount of Uranium and Thorium on Earth was fixed when the Earth was formed, and one might consider nuclear fuels less renewable than other fossil fuels, as oil and coal is generated from organic material continuously.
The most important Uranium reserves are found in Kazakhstan, Canada and Australia where Uranium is found in high enough concentrations to be commercially viable at current Uranium prices. Over half the World's yearly Uranium production originates in these three countries. [5] Uranium is found in the form of Uranium oxides and is usually extracted by open-pit mining. The Uranium ore is then purified, and in most cases enriched to obtain a higher percentage of U-235. The Uranium can then be used to manufacture fuel rods, which can finally generate electricity in nuclear reactors. The OECD estimates that there are 6.3 million tons of identified Uranium supplies recoverable at a rate less than $260/kg. At 2008 rates of Uranium consumption, these supplies would last about 100 years. If the projected discoveries are included as well, world supplies will last 220 years. [5] If Uranium consumption rates increase, as they are projected to, the supplies will last even shorter. A long-term energy solution can therefore not be based on minable Uranium in current reactor technology.
The estimates given above only include mineable Uranium, and they also assume that there are no improvements in reactor technology. The most direct way of increasing nuclear fuel supplies would be to extract Uranium from sea water, where it is found in small concentrations. Pilot projects in Japan have estimated the price at 200-300$/kg. [12] The total amount of Uranium in the ocean is about 4.5 billion tons, but it remains to be seen whether it can be extracted on a large scale. Development of breeder reactors where fertile U-238 is transmuted to fissile Pu-239 in-situ such that more fissile material is produced than is consumed, would significantly prolong the lifetime of fissile fuel supplies. The use of the fertile material Thorium in a Thorium fuel cycle could allow us to use the Earth's Thorium supplies. Thorium is three to four times more abundant than Uranium and a Thorium fuel cycle may have significant advantages over the Uranium fuel cycle currently employed. However, significant technological and economic challenges remain before Thorium nuclear reactors are commercially viable and only India has a Thorium nuclear power program. [8] Reprocessing of spent fuel is a currently available technology increasing the efficiency of nuclear fuel by extracting the remaining fissile material in a spent fuel rod by the PUREX method. All major nuclear powers except the United States have a currently operating reprocessing program for spent fuel rods. [13]
The fossil nature of nuclear fuel should not be overlooked when planning for future energy production, but there are many potential solutions to the fuel problem.
© Nils Johan Engelsen. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] M. L. Wald, "U.S. Supports New Nuclear Reactors in Georgia ," New York Times, 16 Feb 10.
[2] J. Dempsey, "Germany Extends Nuclear Plants' Life," New York Times, 6 Sep 10.
[3] P. Moore, "Going Nuclear: A Green Makes the Case," Washington Post, 16 Apr 06.
[4] C. Mortished, "Minister Declares Nuclear 'Renewable'," London Times, 31 Oct 05.
[5] International Atomic Energy Agency, Uranium 2009: Resources, Production and Demand, (OECD Press, 2010).
[6] C. Gupta and H. Singh, "Uranium resource processing: secondary resources", p.55, Springer (2003).
[7] A. Jameson, "Uranium Shortage Poses Threat", London Times, 15 Aug 2005.
[8] "Thorium Fuel Cycle - Potential Benefits and Challenges," International Atomic Energy Agency, IAEA-TECDOC-1450, May 2005.
[9] M. F. James, "Energy Released in Fission," J. Nucl. Energy 23, 517 (1969).
[10] K. Salant, "2010 International Builders Show: At home in the future ", The Washington Post, 30 Jan 2010.
[11] E. M. Burbidge, G. R. Burbidge, W. A. Fowler, F. Hoyle, "Synthesis of the Elements in Stars", 29, 547-650, Reviews of Modern Physics.
[12] N. Seko et al., "Aquaculture of Uranium in Seawater by a Fabric-Adsorpent Submerged System," Nucl. Technol. 144, 274 (2003).
[13] "Status and Trends in Spent Fuel Reprocessing," International Atomic Energy Agency, "