 |
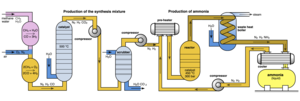
| Fig. 1: Process Flow Diagram for Haber Bosch Process. (Source: Wikimedia Commons). |
The Haber-Bosch process, shown in Fig. 1, is arguably one of the most revolutionary industrial chemical processes ever developed. [1] This process that was created at the turn of the twentieth century uses elemental nitrogen and hydrogen to synthesize ammonia. [2] The hydrogen feed for this process is commonly produced via methane steam reforming and the nitrogen feed is obtained from extracting it from air using an air separation unit at the plant. [2]
Ammonia, the product of the Haber-Bosch process, is used to make inorganic fertilizers. [2] Fertilizers enable farmers to significantly increase their crop productions without having to worry about using more land to compensate for the reduced productivity of the soil overtime. [2] In just one day, crop production uses 300,000 short tons of fertilizers and agriculture produces 19.5 million short tons of cereals, roots, tubers, fruits, and vegetables. [2]
Though nitrogen could and can be obtained from biofixation, atmospheric deposition, and from the recycling of crop residues and animal manures, these sources of nitrogen only add up to about half of the global need. [1] Fritz Haber and Carl Bosch then filled this gap between the requirement for naturally fixed nitrogen and the agriculture requirement to feed the growing world population via the large-scale synthesis of ammonia. [1,2] With more ammonia available to make inorganic fertilizers, people's ability to grow food significantly increased, so the Haber-Bosch process coupled with advancements in technology and health care, enabled the world population to grow from 1.6 billion in 1900 to 8 billion today. [1,3]
From Table 1, we can see that on average the world produces 145 million tonnes or 1.45 × 1011 kg of ammonia per year. [4] The average price of this Haber-Bosch ammonia is $298.6 per short ton or $0.33 U.S. per kg. [4] Now that we know how much Haber-Bosch ammonia is produced around the world and how much it costs, let us now dive into how much energy this process consumes. The Haber-Bosch process is energy and greenhouse gas intensive. Ammonia production via the Haber-Bosch process is estimated to comprise 2% of the world's total energy consumption. [5] For the past 5 years, the world's energy consumption on average has been 5.79 × 1020 J (see Table 1), so this means that the Haber-Bosch process approximately consumes 1.2× 1010 J per year. [5,6] Natural gas accounts for 70% of this energy consumption (8.4 × 1018 J per year), so if natural gas has a chemical potential energy of 5.53 × 107 J per kg, about 1.52 × 1011 kg of natural gas is consumed per year in this process. [5,7] We may ask: How much grid electricity is consumed by Haber-Bosch process? Well, grid electricity accounts for only 3% of the process' energy consumption. [5] This amounts to 3.6 × 1017 J per year.
|
||||||||||||||||||||||||
| Table 1: Amount of ammonia produced, average price of Haber-Bosch ammonia, and world's total energy consumption from 2017 - 2021. [4,6] |
The information above is important because first, one can begin to see that if the world were not able to meet the energy demand of the Haber-Bosch process, present fertilizer suppliers of the world would not be able to operate, resulting in the world's food supply collapsing and potential mass famine. Furthermore, we can see that there can be opportunity to incorporate other sources of energy (either renewable or nonrenewable) in the process so that there is not a strong dependence over one specific source. In Table 1, notice that in 2021, the price of ammonia almost doubled. This was because of the higher natural gas prices during this time. [4] As we have seen, natural gas is the main source of energy in this process. [5] Natural gas prices then dictate ammonia prices which can be detrimental to countries who may have issues obtaining natural gas. For instance, Germany is one of the European countries who have largely depended on Russian gas supplies for decades. [8] The Nord Stream 1 and 2 gas pipelines were designed to bring gas from Russia to Germany via the Baltic Sea. [8] With the war in Ukraine, though, Russian supply of natural gas in Germany has stopped, causing the price of natural gas to soar. [8,9] These prices are threatening to leave permanent scars on Germany's manufacturing sector. [9] Energy-intensive sectors, such as chemicals, glass, and metal producers, have fared even worse, declining by more than 2% from July 2022. [9] Many companies are then resorting to moving abroad. [9] This just goes to show how important it is to try to diversify the energy inputs in the Haber-Bosch process; do not want to create situations for companies where it can become impossible for them to operate.
Part of the reason why the Haber-Bosch process is very energy intensive is due its harsh reaction conditions. The reaction runs at temperatures in the range of 400°C-500 °C, pressure in the range of 150-300 bar, and with the presence of an iron-based catalyst. [10] Unfortunately, the iron-based catalysts used in industry are effective for ammonia synthesis above 350°C, so the maximum ammonia yield is at most 30-40%, despite the excess pressurization accompanied by large energy consumption. [11] For this reason, researchers have been investigating efficient catalysts for ammonia synthesis at lower temperatures. [11] Recently, Hattori et al. reported a new approach for low temperature ammonia synthesis that uses a stable electron-donating heterogeneous catalyst, cubic CaFH, a solution of calcium fluoride and calcium hydride formed at low temperatures. [11] Along with research in catalysis, there has also been research groups exploring electrochemical ammonia synthesis using renewable energy. [2] I am very excited for even the smallest research advancement or optimization that can be incorporated in the large scale production of ammonia because this small improvement can contribute lowering the overall energy consumed and greenhouse gases emitted by the Haber-Bosch process.
© Luis Jimenez. The author warrants that the work is the author's own and that Stanford University provided no input other than typesetting and referencing guidelines. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] V. Smil, "Detonator of the Population Explosion," Nature 400, 415 (1999).
[2] G. Hochman, A. Goldman, and F. A. Felder, "Alternative Ammonia Production Processes and the Use of Renewables," in Green-Economy: Systems Analysis For Sustainability, ed. by G. S. Murthi et al., (Elsevier, 2021).
[3] D. Victor, "World Population Reaches 8 Billion, U.N. Says," New York Times, 15 Nov 22.
[4] "Mineral Commodity Summaries," U.S. Geological Survey, 2019, 2020, 2021, 2022.
[5] "Ammonia Technology Roadmap,"International Energy Agency, October 2021.
[6] "BP Statistical Review of World Energy 2022," British Petroleum, June 2022.
[7] R. B. Laughlin and S. W. Freund, "Economics of Hydrogen Fuel," in Machinery and Energy Systems for the Hydrogen Economy, ed. by K. Brun and T. Allison (Elsevier, 2022).
[8] H. Ellyatt, "German Minister Criticizes U.S. Over 'Astronomical' Natural Gas Prices," CNBC, 5 Oct 22.
[9] A. Cooban, "Rocketing Energy Costs Are Savaging German Industry," CNN, 7 Oct 22.
[10] S. Ghavam et al., "Sustainable Ammonia Production Processes," Front. Energy Res. 9, 580808 (2021).
[11] M. Hattori et al., "Solid Solution For Catalytic Ammonia Synthesis From Nitrogen and Hydrogen Gases at 50°C," Nat. Commun. 11, 2001 (2020).