 |
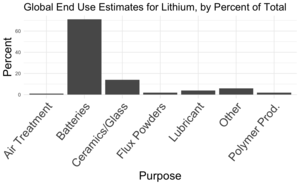
| Fig. 1: TSGS global end uses market estimates for Lithium, given by percent. [5] Batteries are the largest end use for lithium by far. (Source: H. Schwager) |
While lithium is an abundant element on our planet, its importance in both industry and technology make lithium key to our future in clean energy, with the United States labeling lithium as one of its critical minerals in 2018. [1]
Lithium has a number of advantageous properties that make it useful. It is not only the lightest naturally occurring metal but also the least dense solid element on Earth. [2] Lithium possesses the highest specific heat (3.56 J g-1 °K-1) of all the solid elements, and it is more electronegative than any other alkali metal. [3] These factors combine to make lithium an ideal material for our use in technology as we look to find materials with strong energy conduction and efficiency.
However, before we can use lithium in our production and manufacturing, we must first go through the process of harvesting it. With only one valence electron in its outer shell, lithium is highly reactive. Ttherefore, it cannot occur as a pure metal in our Earth, instead occurring in salts and minerals that must be manipulated and mined to achieve the needed product of lithium. [3]
One can think of lithium production in two stages. First, lithium exists naturally in brine and rock and must be mined. Second, the mined product must then be chemically manipulated to extract lithium in our desired form. Lithium can be produced to take the form of lithium carbonate, lithium hydroxide, lithium concentrate, or lithium chloride. [4]
Finally, this mined and chemically changed product can be used in our manufacturing and production. While lithium certainly has a clear role in our world's future in technology, it is important understand our current demand for lithium, how it will grow, and how much lithium our world has.
Lithium historically has been utilized in a wide variety of industries such as glass-working, metallurgy, ceramics, medicine, and industrial production. [3] However, today, lithium's end use is dominated by its use in the modern lithium battery (see Fig. 1).
Scientists throughout the 20th century toyed with creating a powerful lithium battery. Ultimately John Goodenough was responsible for creating the modern lithium-ion battery we see today. [3] Lithium-ion batteries are lightweight, are fast charging, and have a high energy density. [3]
The lithium-ion battery is used in almost all our portable devices, but its most important use at the moment is in electric vehicles. In 2017, batteries made up 39% of global lithium end use. [2] Fig. 1 shows the U.S. Geological Survey's 2021 estimate of the breakdown of global end uses for lithium. Batteries are the greatest use case for lithium by far, with 71% of global lithium product used for batteries. [5] With the rise of EVs and continued production of portable electronics, global demand for lithium is booming. These technological advances are driving demand for lithium.
While lithium naturally occurs in trace amounts throughout the environment, the majority of the world's industrial lithium supply comes from mineral-rich brines often located beneath salt flats and from hard rock minerals. Its density is sufficiently high in these sources to merit economically feasible extraction. [3] For hard rocks, the most common source of lithium can be found in a subset of rocks called pegmatites. [3] Because brines contain high concentrations of lithium and are less expensive to extract from than mineral rocks, mineral-rich brines beneath high altitude salt flats produce the largest percentage of our world's lithium. In 2018, the world's lithium resources were made up of lithium from 59% mineral-rich brines, 25% hard rock minerals, and 16% other sources such as clay, oilfield brines, and geothermal waters. [3] Fig. 2 shows a salt pan in Argentina known as Salar del Hombre Muerto where lithium is harvested.
Geographically, world lithium supplies are highly concentrated in a handful of regions, with Bolivia, Argentina, China, and Australia, producing more than 92% of global lithium from rock and brine. [4] In these countries, lithium manufacture and export play a huge role in the economy. The United States receives 55% of its annual imported lithium from Argentina, 36% from Chile, 5% from China, and 4% from other sources. [5]
 |
| Fig. 2: Lithium brine mine at Salar del Hombre Muerto in Argentina. (Source: Wikimedia Commons) |
Because lithium is a finite natural resource, it is worth noting our current understanding of the total amount of lithium our planet contains. First, we must draw the distinction between lithium resources and lithium reserves. Lithium resources means lithium estimated to exist within our Earth that may be feasibly extracted at some point in the future. Lithium reserves refers to lithium identified that is economically feasible to extract today. [6] Further, both numbers are changing. As the value of lithium increases, more effort is put toward identifying new sources of lithium; as the price of lithium increases, more resources become economically viable and are then classified as reserves. The U.S. Geological Survey's Mineral Commodities Summary of 2021 reports that globally, there are an estimated 86 million metric tons of lithium resources and 21 million metric tons of lithium reserves. [5]
In the way of annual demand, the U.S. Geological Survey reports that in 2020, global consumption of lithium totaled 56,000 tons of lithium product. [5] This number is largely the same as the amount of lithium consumed in 2019. It is believed that consumption did substantially increase from 2019 to 2020 because of decreased demand due to the Coronavirus Pandemic. Even so, in the coming years, demand is expected to increase significantly. One study from Yale University conservatively projects that by 2100 global lithium demand will reach 4.4 million metric tons of Lithium Carbonate Equivalent per year, which translates to 827,220 tons of lithium product. [6] As we continue down the path of electric vehicles and greener electronics, lithium will be key to our success. Although our current lithium reserves dwarf today's global demand, as our demand for lithium rapidly increases, this will not always be the case. Under the projected demand in 2100, our current reserves would only last 25 years. We must look for ways to sustainably use our lithium reserves and to be able to shoulder the heavy demand that is coming for lithium.
© Haley Schwager. The author warrants that the work is the author's own and that Stanford University provided no input other than typesetting and referencing guidelines. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] "Final List of Critical Minerals 2018," 83 Fed. Reg. 23295 (May 18, 2018).
[2] L. Gil-Alana and M. Monge, "Lithium: Production and Estimated Consumption. Evidence of Persistence," Resources Policy 60, 198 (2019).
[3] L. Kavanagh et al., "Global Lithium Sources Industrial Use and Future in the Electric Vehicle Industry: A Review," Resources 7, 57 (2018).
[4] D. Calisaya-Azpilcueta et al., "Assessment of the Supply Chain Under Uncertainty: The Case of Lithium," Minerals 10, 604 (2020).
[5] "Mineral Commodity Summaries 2021," U.S. Geological Survey, January 2021.
[6] H. Ambrose and A. Kendall, "Understanding the Future of Lithium: Part 1, Resource Model," J. Ind. Ecol. 24, 80 (2020).