
 |
| Fig. 1: The 100F Reactor at Hanford, Wash., begun in December 1943 and completed in December 1944, one of three such Hanford reactors which made up the Nation's first large-scale plutonium production capability. (Source: Wikimedia Commons). |
Plutonium isn't found in the natural world. It is a radioactive metallic man-made element with chemistry closely relating to that of Uranium. In 1941, Abelson and McMillian discovered the first transuranic element Neptunium, element 93. [1] Noticing that Neptunium was a β-emitter with a short half-life and that its decay was followed by no further emission of radiation, Glenn Seaborg, a young assistant professor at the time, correctly deduced that neptunium must be followed in the periodic table by another element with a long half-life, element 94, later called Plutonium. [1] Because Plutonium was chemically different from Uranium but also fissionable, the wartime leaders of the Manhattan Project decided in 1941 that chemical separation might be easier to achieve than isotopic separation. [1] In the end, both were accomplished, but the great urgency at the time, as the world was in a war, dictated that alternate strategies be tried simultaneously. [1] This meant that Seaborg had to isolate large quantities of a man-made element whose chemistry was unknown and separate it from a number of highly radioactive fission products, also with largely unknown chemistry. [1]
Just north of Richland, Washington, sits the site of a crucial part of the Manhattan Project, along with Los Alamos and Oak Ridge: the Hanford nuclear facility, as seen in Figure 1. Each of these three top-secret locations performed a distinct function: Los Alamos worked on designing nuclear weapons, Oak Ridge separated U-235 from U-238, and Hanford manufactured Pu-239 from U-238. [2] Ultimately, most of U.S. nuclear arsenal depended on the plutonium produced at Hanford. The Hanford site was built on what was approximately 1700 km2 of farms and towns. [2] The first reactor, the 250 MW B Reactor, was finished in September 1944 and immediately began irradiating U-238 atoms with neutrons to make Pu-239. [2] The first 8 km2 separation plant was finished a few months later, where the plutonium would be chemically extracted from the leftover uranium and other fission products (which were then put into waste tanks). [2] Together, this was the first large-scale plutonium production facility ever built. Two more reactors were built at Hanford before the end of World War II. Eventually, at the height of the Cold War in 1963, Hanford was home to thousands of buildings, including nine reactors and five separation plants of various designs. [2] Hanford continued to manufacture Plutonium at full capacity through the mid-to-late 1960s, and produced over 67.4 tonnes of Plutonium in its lifetime at Hanford, making it one of the largest Plutonium manufacturing facilities in U.S. history. [2]
To create Plutonium as a distinct chemical element, a scientist has to bombard natural uranium (U-238) with neutrons; it can then be separated from Uranium by a series of chemical reactions. Fast neutrons slowly lose kinetic energy. If they primarily bouncing off of heavy fuel particles (i.e. uranium and plutonium), they slows down in small increments - making it increasingly probable that the neutron will be captured and consumed by certain fuel species. [3] This will, in turn, create a fissionable Pu-239 species. This Plutonium is now a fuel particle that has been created, a new fissionable target for the neutrons that will contribute to the overall "fast" neutron population. [3] After some level of depletion of the fissile material, the fuel is reprocessed, whereby the existing fissile material (namely Uranium and Plutonium) is separated out from the neutron capturing material (that which works against sustaining a neutron population within the reactor). [3] This can then be sent back into the reactor core for further orradiation. This process can be efficient in terms of making more use out of the nuclear fuel resource; however, there are issues with regards to weapons production and nuclear proliferation due to the separation of Plutonium. [3] Most Plutonium samples contain the isotopes Pu-239, Pu-240., Pu-241 u, and Pu-242. Am-241 and U-237 are always present as decay products of Pu-241. [4] An accurate measurement of Plutonium isotopic composition is usually required to interpret the results of neutron coincidence or calorimetry measurements. Several methods have been developed for determining Plutonium isotopic composition by gamma-ray spectroscopy. [4] Finally, once Plutonium is in its purest form, it can be ready to create nuclear weapons.
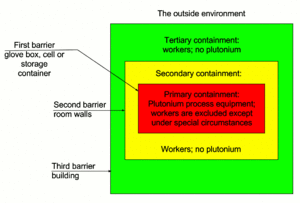 |
| Fig. 2: Schematic drawing of three-physical-barrier static containment. (Source: E. Morris, after the IAEA. [5]) |
Plutonium is highly reactive and requires serious care and storage when handling under any circumstance. Plutonium facilities should be divided into a number of operating zones by using barriers to separate areas of different risks from radiation and contamination. [5] In practice, according to the IAEA, containment is achieved by barriers (three barriers of static containment) and air flow using ventilation systems to establish pressure gradients (dynamic containment). [5] At least one static containment barrier (normally the primary containment) must be provided between the plutonium source and the workers. To ensure containment integrity, facilities and equipment are designed to avoid fire and explosion, remove heat, and prevent mechanical failure (including drop of load) that may threaten containment integrity. [5]
In addition, monitoring is carried out continuously to detect any failure of containment. Inhalation of a large amount (> 105 Bq, or 2.7 μCi) of insoluble Plutonium activity, such as might occur in a fire or explosion, can result in serious acute health effects. [5] Preventative measures become essential and can be considered in three categories: (1) controlling ignition sources, (2) controlling the use of combustible materials, and (3) reducing inventory involved in a fire. In a Plutonium facility, the main risk of explosion is linked from the use of solvents and diluents, and to the use of hydrogen by radiolysis. In addition, the criticality risk is analyzed early in the design of a Plutonium plant, as seen in Figure 2; criticality can be affected by mass, shape, volume, moderation interaction, neutron absorption, reflection and density. [5] Criticality prevention measures will affect the choice, design and layout of equipment. Criticality is avoided by using one of the five methods: 1) restricted geometry, 2) mass and moderation control, 3) limitation of concentration of fissile materials, 4) neutron absorbers, 5) spacing of equipment. [5] These and many other concerns should not be taken informally when dealing with Plutonium, the environment, and the health of the operators at the plant.
Without plutonium's discovery, scientists would not have encountered a unique and revolutionary opportunity to help the world. Production was essential for the Manhattan Project and advancing techniques for isotope separation. It is amazing that elements like plutonium exist in nature, and that mankind has determined how to utilize their scientific power.
© Emma Morris. The author warrants that the work is the author's own and that Stanford University provided no input other than typesetting and referencing guidelines. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] D. E. Koshland, "Glenn Seaborg (1912-1999)," Science 284, 447 (1999).
[2] E. Eason, Hanford's Hot Tanks," Physics 241, Stanford University, Winter 2011.
[3] G. Roberts, "Nuclear Reactor Basics and Designs for the Future," Physics 241, Winter 2013.
[4] D. Reilly et al., eds., "Passive Nondestructive Assay of Nuclear Materials," U.S. Nuclear Regulatory Commission, NUREG/CR-5550, March 1991.
[5] "Safe Handling and Storage of Plutonium," International Atomic Energy Agency, STI/PUB/1061, September 1998.