 |
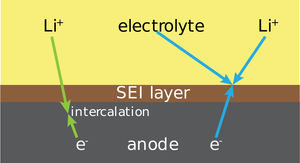
| Fig. 1: Schematic representation of the SEI layer between the anode and electrolyte. The layer is permeable to positive lithium ions but not to electrons. (Source: Wikimedia Commons) |
Increasing global energy demands necessitate improved energy storage methods, a promising one being lithium-ion batteries. Lithium metals low electrochemical potential and high theoretical capacity make it a desirable material for use in high energy density battery applications, from consumer electronics to military power sources. [1] Lithium-ion cells function through the reversible intercalation of lithium ions between the two battery electrodes while electrons are sequestered through an external circuit and used for power generation. Although promising, barriers in fabrication, including optimizing electrode materials and kinetic inefficiencies, present obstacles to unlocking the full theoretical potential of lithium-ion batteries.
CNTs are an allotrope of carbon composed of either single or multiple sheets of graphene rolled into a cylinder. Their unique properties are attributed to the quantum effects caused by their high aspect ratio: diameters range between 0.2 - 5 nm and lengths from 10 nm - 1 cm. In addition, the direction around which CNTs are rolled, which dictates their specific structure, causes CNTs with different structures to exhibit different properties (see Fig. 1). Leveraging their properties, including high mechanical strength, elasticity, surface-to-volume ratio, thermal conductivity, and electron mobility, may improve current and potential energy conversion and storage applications.
During charging and discharging, lithium ions shuttle between the cathode and anode through an electrolyte, the battery component that separates the two electrodes and is conductive to ions, but not to electrons. Lithium, a highly reactive metal, initially decomposes at the contact with the electrolyte to form the solid electrolyte interphase (SEI) (see Fig. 1). [2] This layer, about 30-50 nanometers thick, passivates the lithium electrode and prevents more lithium metal being consumed by reactions with the electrolyte. However, it is vastly complex and difficult to analyze, and although it has been studied extensively, it is one of the least understood components of the battery. [3] Composed of a network of organic and inorganic molecules, the SEI is fragile and reduces the cycle life of the cell due to its irreversible use of lithium and reaction inefficiency. [2] Effective SEIs require mechanical stability to ensure it does not break with changing electrode volume, electronic insulation to prevent further consumption of the lithium electrode, and ionic conduction to allow the transport of lithium ions between electrodes, and further research must be done to determine how to understand and optimize these properties.
Studies of SEI morphology and chemistry have employed numerous surface sensitive techniques, including X-ray spectroscopy, electron microscopy, and nuclear magnetic resonance spectroscopy. [3] These characterization techniques have specific sample preparation requirements, and often must be performed ex situ. These requirements limit the scope of study, as SEIs are highly affected by changes in environment, and an external environment does not accurate represent how the SEI functions within a cell. In situ experiments of neutron reflectometry (NR), atomic force microscopy (AFM), and X-ray reflectometry have been performed and have illuminated key insights on the early formation of the SEI. In situ NR revealed the formation of a lithium-rich layer and the rate of growth based on electrode voltage, and these results were confirmed with the other methods.
Another study investigated the change in chemical composition of the SEI using soft X-ray absorption spectroscopy (XAS). [4] The results show that when using a carbonaceous anode material as a scaffold to hold lithium, a reversible formation of the SEI was possible between the electrode and electrolyte. By analyzing the absorption spectra of lower energy soft X-rays, researchers tracked the complex chemical species in the SEI and determined that some amount of the layer is decomposed upon discharging of the cell.
Lithiums high gravitmetric and volumetric energy densities make it a highly desirable electrode material for energy dense batteries. Lithium-ion batteries can be used in a range of applications, and thus have been researched extensively. Even so, current technologies have not reached theoretical capacity limits due to kinetic losses in efficiency. The SEI plays a critical role in the performance of lithium-ion batteries and, although studied significantly, is still not well-understood. Recent studies on the morphological and chemical structure of the SEI have resulted in findings that are the first step in unveiling the nature and behavior of the SEI layer. This in turn will improve electrode and electrolyte designs to optimize the function of lithium-ion batteries. Thus, understanding and improvement of the SEI will have a significant impact on new-age battery technologies.
© Kelsey Pian. The author warrants that the work is the author's own and that Stanford University provided no input other than typesetting and referencing guidelines. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] M. K. Gulbinska, ed., Lithium-Ion Battery Materials and Engineering: Current Topics and Problems from the Manufacturing Perspective (Springer, 2014).
[2] S. Jurng et al., "Effect of Electrolyte on the Nanostructure of the Solid Electrolyte Interphase (SEI) and Performance of Lithium Metal Anodes," Energy Environ. Sci. 11, 2600 (2018).
[3] M. Steinhauer et al., "In Situ Studies of Solid Electrolyte Interphase (SEI) Formation on Crystalline Carbon Surfaces by Neutron Reflectometry and Atomic Force Microscopy," ACS. Appl. Mater. Inter. 9, 35794 (2017).
[4] Y. Kim et al., "Revisiting Solid Electrolyte Interphase on the Carbonaceous Electrodes Using Soft X-ray Absorption Spectroscopy," ACS. Appl. Mater. Inter. 10, 29992 (2018).