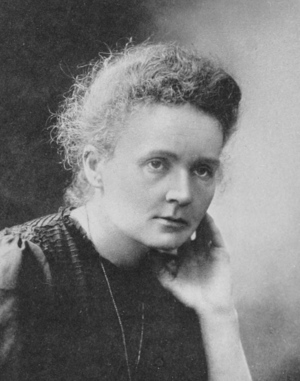
 |
| Fig. 1: Portrait of Marie Curie after winning the Nobel Prize in Chemistry. (Source: Wikimedia Commons) |
November 7, 1867, one of the most famous female physicists, Marie Sklodowska, was born in Warsaw, Poland. [1] One of seven children born to teachers, Marie showed an interest in mathematics and physics, which her father taught at a high school. [1] Universities in Poland did not allow women to attend, so in 1891, Marie moved to Paris to attend Sorbonne. By 1894, she had graduated at the top of her class with degrees in both mathematics and physics, taking after her father. [2] While in attendance, she met Pierre Curie, a professor at the university. On July 26, 1895, Marie married Pierre and remained in Paris to conduct research alongside him. [2]
Early in her career, Marie took an interest in Becquerel rays. She did not have the funding for a lab, so she conducted her research in a storeroom. In the tight working quarters, she discovered that the chemical or physical state of the uranium did not matter. The more uranium in the compound, the more radiation it gave off. [1] She continued exploring other types of compounds that emitted Becquerel rays and found that thorium also produced rays similar to uranium. She decided to name the activity of the two compounds radioactivity. [1] After this discovery, she and Pierre turned their attention to a mineral called pitchblende (uranium oxide), which is about 70 percent uranium, but more active than uranium. [3] They assumed there was a radioactive substance inside pitchblende causing this. Through fractional crystallization, they were able to discover polonium and radium. [1] With the new knowledge of these two substances, Curie later realized that radium could be used as a gamma ray source on X-ray machines. This was extremely useful during World War I because it allowed for smaller, more accurate x-ray machines that could be used by medics. [3]
In 1903, following her and Pierres research on Becquerel rays, they both received the Nobel Prize for Physics. Pierre died in a car accident in 1906, but Marie continued her research. She went on to receive the Nobel Prize for Chemistry in 1911 for her work on radioactivity (see Fig. 1). She was the first woman to receive a Nobel Prize and remains the only woman to receive a Nobel Prize in two different fields of research. [1]
Throughout her work with radium, Marie was unaware of the effects of radioactivity exposure on the body. In her lab, she would keep tubes of radium in her pocket. [3] She began to suspect that radium negatively impacted health when one of her fellow researchers died of a blood disease, and then a few years later her personal assistant died of a blood disease. Even though she suspected that radium exposure was bad for her health, she did very little to monitor her own blood. In 1932, she broke her wrist and the break took much longer to heal than it should have. She then began to notice that her vision was deteriorating and radiation burns on her fingers were becoming more and more painful. Some days she felt too ill to even go to the lab, and finally on July 4, 1934, Marie died from aplastic anemia. [1]
Marie suspected that her health was being negatively impacted by radium exposure, but did nothing about it, most likely because there weren't any effective treatments for radium poisoning yet. At the time, scientists knew that radium was metabolized like calcium. In an attempt to remove it from the system, they manipulated calcium intake. [4] This caused little to no improvements, so parathyroid hormone was added to the treatment. Again, there was some reduction of radium, but not a significant amount. It wasn't until after Marie's death that they realized once radium is in the bones, it is extremely difficult to extract. The lack of therapies for radium exposure may explain why Marie just ignored her symptoms, because she was fully aware of her fate. [4]
© Jenna Gray. The author warrants that the work is the author's own and that Stanford University provided no input other than typesetting and referencing guidelines. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] N. Pasachoff, Marie Curie: And the Science of Radioactivity (Oxford University Press, 1997), pp 10, 37, 42, 102.
[2] M. Ethridge, Marie Curie: Radioactivity Pioneer and the First Woman to Win a Nobel Prize (Cavendish Square Publishing, 2017), pp 10, 14.
[3] M. Caballero, "Marie Curie and the Discovery of Radioactivity," Physics 241, Stanford University, Winter 2016.
[4] J. N. Stannard, "Radioactivity and Health: A History," U.S. Department of Energy, DOE/RL/01830-T59, October 1988.