 |
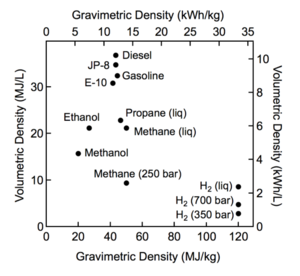
| Fig. 1: Comparison of specific energy (energy per mass or gravimetric density) and energy density (energy per volume or volumetric density) for several fuels. (Courtesy of the DOE) |
Hydrogen fuel cell vehicles (FCV or FCEV) offer an alternative to gasoline-powered cars while avoiding the extended recharging times required of most electric vehicles. Hydrogen is an excellent energy vector as it is abundant, burns cleanly, and has a high gravimetric density (energy per mass) as seen in Fig. 1. However, its energy density (energy per volume) is very low at ambient temperatures, making hydrogen storage a significant issue for mobile applications. [1] Most current technologies are focused on storing hydrogen at high pressures, but this is both expensive and insufficient. For example, to drive 300 miles, an FCEV would need ~5 kg of hydrogen. At 700 bar (which is the capacity of the standard pressure vessel) a storage system would have a volume of 200 L (>3x the volume of a gasoline tank). [2] One area which has been receiving a lot of attention recently is materials-based methods of hydrogen storage in which the hydrogen is stored on the surfaces of (adsorption) or within solids (absorption). [3] This article shall focus on the use of LaNi5H6 and similar alloys as a storage system for hydrogen where LaNi5H6 is an intermetallic hydride with hydrogen atoms in its interstitial spaces. [4]
Fig. 2 demonstrates the general mechanism by which a metal hydride system stores hydrogen. The molecular hydrogen dissociates at the surface and intercalates into the metal matrix during absorption and two H atoms recombine to H2 during desorption. Thus, hydrogen atoms are located on the interstitial sites of the LaNi metal lattice. [5] This lattice expands during absorption causing strains and lattice defects which means brittle metals can not be used in the lattice on their own. LaNi5 is particularly promising due to its ability to absorb and desorb hydrogen quickly within required temperature and pressure ranges and with little hysteresis which allows for a good cycle life. [2,6] Namely, it can be filled and emptied many times without any noticeable decrease in capacity or performance. Furthermore, it is capable of storing this hydrogen at a fairly low pressure, making it an excellent candidate for mobile applications where high pressure systems become dangerous and expensive. However, hydrogen in LaNi5H6 only has a mass density of 2% meaning that the vast majority of the system is made up of La and Ni. This is insufficient for mobile applications, where 4-5 mass % is required (6.5 mass % and 62 kg H2 m-3 are the targets of the US DOE). [5] However, in the next section we will begin looking at other materials which share the benefits of LaNi5 but can store a larger mass % of hydrogen.
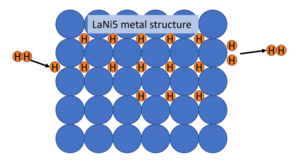 |
| Fig. 2: Visual representation of intercalation of hydrogen in a metal hydride. (Source: I. Naccarella. After Schlapbach and Züttel. [5]) |
Further research has looked beyond LaNi5 to other metal alloys with similar crystal structures but which allow for higher mass densities of hydrogen. In fact, Philips Research recently found that a fluorite type of Mg-based compound can store up to 4x the amount of hydrogen as LaNi5, making this compound an attractive alternative for high energy storage systems and a way for the industry to meet the target of 6.5 wt.% hydrogen storage set by the US Department of Energy. [6] Furthermore, several other materials such as Mg-based metal alloys have surpassed LaNi5-based alloys recently in this regard although they still have issues regarding operating pressure and ease of extracting the H2. Thus, LaNi hydride systems still have advantages in quick absorption and desorption which is critical for use in fuel cell vehicles.
Thus, we can see that LaNi5H6 and similar systems represent an exciting frontier in hydrogen storage for mobile applications. Despite their many advantages however, they all have issues with the amount of hydrogen they can store relative to the amount of metal alloy. Future work should be focused on this area and should explore other metal alloys with similar crystal structures but with smaller atoms. Progress has already been made in this area and it will be exciting to see how it progresses over the next few years or if a competing storage technology overtakes it.
© Ian Naccarella. The author warrants that the work is the author's own and that Stanford University provided no input other than typesetting and referencing guidelines. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] A. Contryman, "Hydrogen Storage Technology," Physics 240. Stanford University, Fall 2010.
[2] R. J. Westerwaal and W. G. Haije. "Evaluation Solid-State Hydrogen Storage Systems," Energy Center of the Netherlands, ECN-E-08-043, April 2008
[3] K.-H. Young and J. Nei, "The Current Status of Hydrogen Storage Alloy Development for Electrochemical Applications," Materials 6, 4574 (2013).
[4] A. Züttel, "Hydrogen Storage Methods," Naturwiss. 91, 157(2004).
[5] L. Schlapbach and A. Züttel, "Hydrogen-Storage Materials For Mobile Applications," Nature 414, 353 (2001).
[6] C.-Y. Hsieh et al., "Developing Hybrid-Power Fuel Cells with a Low Pressure Hydrogen-Storage System Used in an Electric Forklifts," Int. J. Electrochem. Sci. 12, 6266 (2017).