
 |
| Fig. 1: Flaring in the Bakken. The flame can readily be seen at a distance at night. (Source: Wikimedia Commons) |
Modern society makes great use of hydrocarbons and commodity chemicals, requiring the drilling of wells for hydrocarbons, and refineries and chemical plants to produce the products that drive our society. In the course of operation of these complex machines, flaring will occur. Flaring is the burning of waste gasses through a flare stack or other combustion device. [1] For a sense of scale, 165 billion cubic feet of natural gas was flared in 2011. [2] It is different from a furnace or other form of combustion in that it is not intended to be a feature of the plant for profit, but rather for safety. In Fig. 1 is an image of flaring in action.
In general, flaring is for pressure relief without simply venting dangerous chemicals to the environment. Flaring often occurs during discovery and testing of an oil well. During operation of a well, it can be that there are gases which are not economical to transport or capture, and so are flared. It can also occur during chemical processing. In chemical processing, flaring is usually to remove waste products or for emergency pressure relief. Start-up and shutdown of plants are also common sources of flaring; the list of possible examples is long. [1]
Products of flaring are often water and carbon dioxide with usually greater than 98% combustion of volatile organic compounds. [3] This is excellent from a toxicity standpoint. Depending on the inlet gasses, other products such as sulfur dioxide are possible, which can be locally restricted. [4] Flaring can be used in place of venting waste streams to reduce the load of toxic or greenhouse gasses, such as methane, which is more than 20 times as deleterious as carbon dioxide towards global warming, where carbon dioxide is a direct combustion product. [5] In this way, flaring is far more acceptable than venting, or simply releasing chemicals into the atmosphere.
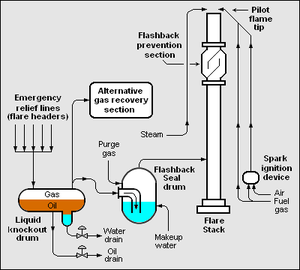 |
| Fig. 2: Diagram of a flare stack. (Source: Wikimedia Commons) |
Basic description of units for flaring:
In Fig. 2 is an example of a flare attached for an industrial plant. Here can be seen an inlet flow for pressure release, coupled to a knockout drum for vapor-liquid separation, that liquids like oil and water will not be drawn into the flare stack and can be recovered. The diagram includes an "Alternative Gas Recovery Section" for the purpose of recovering some of the gas to be flared for use; this may not be sufficient to contain all inlet gas, or may not be present. In this diagram, the inlet gas to be flared continues to a flashback seal drum, which has two important mechanical characteristics. First, there is a threshold pressure required for combustible inlet gasses to be able to pass from the knockout drum to the flashback seal drum. Second, the influx of purge gas removes any lingering combustible gasses. Both of these characteristics make it challenging for any flame to travel down the flare stack and into the refinery, causing destruction. The flare stack has a flame for ignition at the top, and in this case is designed to minimize flashback. Here, there exists steam injection to improve the quality of combustion. [4,6]
Flares are implemented with concerns such as gas composition and pressure, safety, environmental regulations, and social effects (noise and light pollution). Also considered are the effects of thermal radiation, temperature, wind shear, and efficacy of combustion. [4,6]
Flaring represents a large loss of otherwise valuable products and energy capacity, as well as contributions to global warming. [2,7] Attempts to minimize flaring and associated losses and effects are ongoing. [1,7] Redesigning or modifying start-up and shutdown processes in chemical plants is one example. [1] Some problems are infrastructure related, especially in the case of flaring at the well itself. [8] Often, small quantities of natural gas are available in the form of associated gas, or natural gas that is liberated in the process of extracting oil from a reservoir, such that the natural gas released is not what drew companies to the well. A report from the North Dakota Pipeline Authority notes that about 29% percent of natural gas flaring in the Bakken Oil Field in March 2013 was due to wells that were not connected to natural gas pipelines or processing facilities, or had insufficient connections. [9-11] A significant opportunity exists to reduce flaring by improving the infrastructure and connecting them to pipelines and plants that can process the natural gas, as opposed to flaring. [7,8] The report identifies several problems with trying to collect this natural gas. Insufficient compression can be one source of failure, where wells of different pressures are connected to the same pipeline, causing flaring in the lower pressure well. Larger pipelines may be needed, to handle the volume required, as well as buildup of liquids found with the natural gas that can begin to pool and take up volume in the pipe. [10] These infrastructure-related problems are significant.
Other solutions to circumvent problems of flaring from wells have been investigated, such as re-injecting the gas into the well it was drawn from. This is particularly attractive in the instance of dealing with high-sulfur streams, thus removing the cost of desulfurization. On-site liquefaction or transformation to value-added chemicals have also been investigated and Emam lists a number of modern attempts. [1,8] However, on-site chemical transformations can be heavy in capital costs and are influenced by economics of scale; this is most likely to work at sites with very large amounts of flaring. [9] On-site electricity generation from flaring has also been examined, but is limited by on-site demand and grid access. [8]
Flaring is undertaken as a way to remove dangerous gasses with lower harm to the environment. It is used in safely regulating pressure in chemical plants, as well as handling natural gas release in wells. Alternatives, such as piping the gas to a plant or on-site capture and use, are of great interest.
© Victor Miller. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] E. A. Emam, "Gas Flaring in Industry: An Overview," Petroleum and Coal 57, 532, (2015).
[2] "Annual Energy Review 2011," U.S. Energy Information Administration, DOE/EIA-0384(2011), September 2012.
[3] "EPA Air Pollution Cost Manual, 6th Ed.," U.S. Environment Protection Agency, EPA/452/B-02-001, January 2002.
[4] N. P. Cheremisinoff, Industrial Gas Flaring Practices (Wiley-Scrivener, 2013), pp. 23-58.
[5] D. Reay, P. Smith, and A. Van, Amstel, Methane and Climate Change (Earthscan, 2010).
[6] A. Bahadori, Natural Gas Processing: Technology and Engineering Design, 1st Ed. (Gulf Professional Publishing, 2014).
[7] M. R. Johnson and A. R. Coderre, "Opportunities for CO2 Equivalent Emissions Reductions Via Flare and Vent Mitigations: A Study for Alberta, Canada," Int. J. Greenh. Gas Con. 8, 121, (2012).
[8] "Oil and Gas Transportation," U.S. General Accounting Office, GAO-16-667, August 2014.
[9] V. Smil, Natural Gas: Fuel for the 21st Century (Wiley, 2015), pp. 100, 179.
[10] J. J. Kringstad, "North Dakota Natural Gas: A Detailed Look at Natural Gas Gathering," North Dakota Pipeline Authority, 21 Oct 13.
[11] E. Scheyder, "Exclusive: Bakken Flaring Burns More than $100 Million a Month," Reuters, 29 J ul 13.