 |
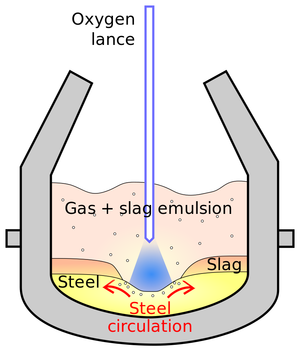
| Fig. 1: Basic oxygen furnace. (Source: Wikimedia Commons) |
The US steel industry has been a cornerstone of American manufacturing since the Industrial Revolution. Andrew Carnegie and Carnegie Steel helped to industrialize the Bessemer process for steel manufacture. With the possibility for newly minted wealth to be had, there was a rise in many steel making companies, all competing against each other to increase their tonnage of steel production. [1] These companies would later be consolidated by J.P. Morgan into the near monopoly of US Steel. While the story of US steel is often one of tenacious industrialists, it is also a story of the utilization of America's vast energy reserves for the creation of manufactured goods. Without the abundant natural energy resources within the US, most notably coal, there would not be a US Steel industry. While early steel manufacturing was predominantly coal powered, modern approaches to steel making have seen a rise in natural gas and electric powered furnaces. Still, coal and coke are significant sources of energy in steel production. On this page, I examine the energy usage in US Steel production. I will review the basic process of creating steel, then outline the various energy sources and amount used in its production. I will then discuss the present and potential futures of energy use in the manufacture of steel and how this may relate to overall energy usage in the US. While the entire production chain, from mining to final manufactured product, adds to the total energy cost of producing steel goods, analysis of the entire chain is too large for this short article. As such, this article will focus primarily on the energy use of raw steel production, from the production of pig iron to the casting of raw steel.
In this section, I will outline the basics of steel production. This is in no way meant to be a full review of the steel production process, but rather a shallow introduction to help describe where in the process energy is used. This section is based on a nice overview of steel production in Fruehan and Section 3 of Worrell et al. [2,3]
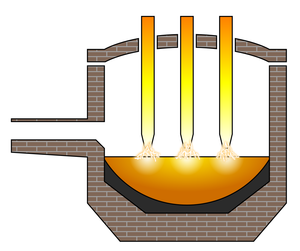 |
| Fig. 2: Electric arc furnace. (Source: Wikimedia Commons) |
Steel is simply low-carbon iron. As such, the steel manufacturing process begins by smelting iron ore (Fe2O3 or Fe3O4) in a blast furnace. This smelting process melts out and separates iron from the original rock material. Iron ore is mixed with coke, a form of very pure coal. The blast furnace burns the coke to heat the iron ore causing it to react into iron (Fe2), nitrogen (N2), and carbon dioxide (CO2). This iron is often known as "pig iron" or "hot metal" as it is a liquid iron that flows from the bottom of the blast furnace. This iron can be used for ironworks or as the starting material for creating steel.
To create steel, carbon is released from the iron through either mechanical means or high temperatures. To understand how we mechanically create steel, imagine a blacksmith pounding on a hot piece of iron, say to create a sword. This pounding not only shapes the object but also gives it strength by forcing out the carbon. Carbon can also be released through high temperatures (~ 1800+ °C). These high temperatures are created by blowing air (and really the oxygen in air) through the iron. The oxygen raises the temperatures and reacts with carbon, creating carbon monoxide (CO). This reduces the carbon content of the iron, producing low carbon steel.
While the "Bessemer process" of steel production is widely known as the defining technology in the mass production of steel, modern steel is manufactured through two primary processes, the "Basic Oxygen Furnace" and "Electric Arc Furnace". [1]
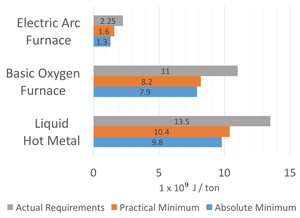 |
| Fig. 3: Energy usage across various steel making processes. [4] (Source: N. Martelaro). |
In a basic oxygen furnace (see Fig. 1), oxygen is blown through liquid pig iron, increasing its temperature and releasing carbon. Pure oxygen is used as it improves the efficiency of the reaction between carbon and oxygen. Hydrocarbon fuel injection (coal, natural gas, oil, and tar) is also used to increase temperature and speed throughput.
In an electric arc furnace (see Fig. 2), scrap steel and solid pig iron is melted using an electric arc. The electric current passes through the steel, heating it a high degree. Since electricity is used to heat the metal, new steel can be created entirely from scrap steel. This avoids the step of creating pig iron from iron ore.
Once the liquid steel is created it can then be cooled, rolled, cast, and formed into a wide variety of products.
Each process in the production of steel uses a certain amount of energy. This section gives an overview of the energy requirements of major production processes including the creation of pig iron, basic oxygen furnace production, and electric arc furnace production. Fruehan et al. have calculated the theoretical minimums for creating steel as well as collected data on practical minimum as and real-world energy use among US steel plants. [4] Fig. 3 shows comparisons of theoretical, practical, and real-world energy requirements for major steel manufacturing processes. Energy is given in units of Joules × 109 per metric ton of steel produced.
|
||||||||||||||||||||||||||||||
| Table 1: Steelmaking energy use (1 × 109 J / metric ton). [4] |
From the data in Table 1 and Fig. 3 it is apparent that the production of hot metal or pig iron is the most energy intensive process for steel production at roughly 13.5 × 109 joules per ton (1000 Kg) of pig iron produced. The basic oxygen furnace is the second most energy intensive process at 11 × 109 joules per ton or steel produced. The Electric arc furnace has significantly less energy required at 2.25 × 109 joules per ton. Finally, the two rolling processes have the least amount of energy required at of below 2.2 × 109 joules per ton.
To understand where these numbers come from, let's estimate the minimum energy for the electric arc furnace. Since the electric arc furnace is using pure electricity to melt steel, we can compute the minimum theoretical energy required based on the energy to heat the steel from the ambient temperature to the melting point. This is given by
where m is the mass of steel we are heating, T is the temperature in Kelvin, and cI is the specific heat as a function of temperature. In addition to the energy required to heat the steel, we must also include the energy required for phase changes. The energy required during a phase change is given by
where m is the mass of steel and H is the latent heat of the phase change. To calculate the total energy used to heat and melt scrap steel we will assume that the steel is pure iron (Fe). During the melting process, iron's specific heat varies greatly from an ambient temperature of 298 °K to iron's melting point of 1811 °K, ranging between 0.45 - 1.5 J °K-1 g-1. Iron also goes through three phase changes during this process, from α -> γ, γ -> δ, and δ -> liquid. Desai has compiled the thermodynamic properties of iron over a range of temperatures and also provides the latent heat for each phase change. [5] Using these values, summarized in Table 2, we can estimate the energy to melt scrap steel:
The python script that performed this integration is available here. This result agrees very well with the theoretical minimum for the electric arc furnace in Table 1 and calculated in Fruehan et al. [4]
|
||||||||||||||||||||||||
| Table 2: Thermophysical properties of iron. |
With an understanding of the energy required for each of the major subprocesses in steel production, we can now discuss how much energy is used to create a steel object. This does assume that the energy input is only in the processes that I have listed above; however, by just looking at the orders of magnitude for each process, we can see that processes of pig iron creation, basic oxygen furnace, and electric arc furnace requires the majority of the energy. Most likely, manufacturing processes further in production will not be as energy intensive as the raw production of steel itself.
Based on the data from Fruehan et al. we can see that raw steel production from iron ore to steel using a basic oxygen furnace will require approximately 24.5 × 109 J per ton of steel produced. [4] This includes the reduction of raw iron ore into pig iron and then conversion of pig iron to steel. Alternatively, energy use with an electric arc furnace is often done with recycled steel rather than pig iron. This means that energy use for electric arc furnace production will be approximately 2.25 × 109 J per ton of steel, 10× less energy than production from raw iron ore. Even if the electric arc furnace is used to melt scrap steel and then the molten steel is reheated in a basic oxygen furnace, you will still on have 15.25 × 109 J per ton of steel. This is about half of the use when creating pig iron and then using a basic oxygen furnace. This, of course, does not take into account the energy that has already been used to create the scrap steel (most likely using the basic oxygen furnace), however, this is still a very useful number for understanding future steel production as it is becoming more difficult to mine high-quality iron ore. One of the benefits of steel is that it is highly recyclable and can be remelted and reused ostensibly forever. This gives a strong reason to recycle any steel possible and gives great motivation for waste management companies to separate and sell steel scrap as raw material for future steel products.
The primary goal of this article is to discuss the basic energy requirements of steel production. However, while energy efficiency may be one goal of production, so too is cost efficiency. The price of steel (at the time of writing this) is about $550 per ton. We can estimate the production costs due to the energy required by assuming that the creation of pig iron and the basic oxygen furnace use coal or natural gas to power the process and an electric arc furnace uses pure electricity.
Coaking coal from the Appilachia region of the US and US natural costs the same at about $2.50 per million BTU or $2.4 × 10-9 per J. [6] Electricity for industrial use is about $0.07 per kWh or $1.94 ×10-8 per J (estimate for Pensylvania). Given these enery costs per joule, we can estimate the costs of production for each process, as shown in Table 3.
|
||||||||||||||||||||||||||||||||||||||||||
| Table 3: Estimated energy costs for steel production. Energy cost. [6] |
From this basic cost analysis, we can see that percentage cost of the energy to produce steel is the same across each process. Even the highest cost of production of pig iron to electric arc furnace production is only 14% of the total market price of steel. Thus, at this time, while there are significant energy savings by using scrap steel and an electric arc furnace to produce new steel, there is little cost savings. The benefits of using electric arc furnaces will mostly come from the use of scrap steel. As high-quality iron ore reserves run out, lower iron content ore will need to be processed to create steel. This may raise energy requirements in the future and could raise costs. For there to be any appreciable difference in the costs of production between energy sources, we will need to see a divergence in the cost per joule. It is possible that natural gas prices will fall below that of coal, making natural gas more affordable. However, at this time, the costs of energy are basically the same across energy sources, most likely due to all these energy sources being ultimately sourced from carbon (electric power plants running on coal or natural gas).
In this article, I have given a basic introduction to steel production and the energy requirements for the production of pig iron, basic oxygen furnace steel, and electric arc furnace steel. From the analysis of the energy requirements for each of the major processes, the electric arc furnace is by far the most energy efficient in producing steel. Overall, the production of steel is highly energy intensive. While one may think that increased energy efficiency may lead to cost savings, at current market rates for coal, natural gas, and electricity, there is little difference in the cost of production. As we move forward and think about alternative energy futures, it will be imperative to better recycle already produced steel. If someday the costs of coal and natural gas become much higher per joule than the cost of electricity per joule, the electric arc furnace will become a more attractive production method. We will then see more and more reliance on the efficient collection of scrap steel and less on the mining of raw iron ore. In the future, we will be mining landfill for steel that was unfortunately tossed out before the introduction of efficient sorting at waste management facilities.
© Nikolas Martelaro. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] A. Cotter, The Authentic History of the United States Steel Corporation (Moody Magazine and Book Company, 1916).
[2] R. J. Fruehan, The Making, Shaping and Treating of Steel (AISE Steel Foundation, 1999) pp. 1-35.
[3] E. Worrell et al. "Energy Efficiency Improvement and Cost Saving Opportunities for the U.S. Iron and Steel Industry," LBNL-4779E, October 2010.
[4] R. J. Fruehan et al., "Theoretical Minimum Energies to Produce Steel," Energetics, Inc., March 2000.
[5] P. D. Desai, "Thermodynamic Properties of Iron and Silicon," J. Phys. Chem. Ref. Data 15, 967 (1986).
[6] "Annual Energy Outlook 2016," U.S. Energy Information Administration, DOE/EIA-0383(2016), August 2016.