 |
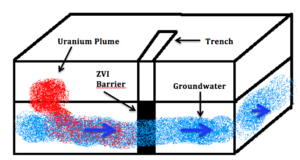
| Fig. 1: Schematic of a zero-valent iron barrier for remediation of uranium in groundwater. (Source: M. Khalaf) |
Nuclear waste disposal continues to be one of the largest challenges in the development of nuclear energy. Currently the majority of nuclear power plants in the US dispose their nuclear waste on-site. This poses a serious long term danger. Currently management options for nuclear waste include near surface-surface disposal (in the order of tens of meters) and deep geological disposal (in the order of hundreds of meters), both onshore and offshore. Moreover different radioactive material requires different management solutions. The U.S. Environmental Protection Agency (EPA) divides nuclear waste into several categories: uranium tailing, low-level, high level and transuranic waste. [1] Each of the above are regulated differently based on their potential harmful effects on the environment and subsequently on human health. Even more urgent than deciding on an appropriate waste site is the remediation of nuclear waste leaks. Groundwater contamination by radioactive material is a notorious example of the consequences of inappropriately disposed nuclear waste. The following paper will explore recent methods used to remediate such environmental catastrophes in waste disposal and mill sites geographically located near aquifers.
Today, the US holds over 230 million tons of uranium mill toiling at mill sites throughout the country. [2] One of the effects of misplaced uranium mill tailing piles is the potential diffusion of the material to nearby sub-surface aquifers. Plumes migrating to these sites have been found to be present in the water on the order of several hundreds parts per billion (ppb). Similarly nuclear waste sequestered underground can leak out and contaminate drinking water reservoirs. The EPA has set a Maximum Contaminant Level for uranium of 30 ppb, a value that is greatly exceeded at the contaminated sites. [3] An interesting question which will not be covered in detail int this paper is: why is the uranium leaking in the first place? In short, underground radioactive waste is traditionally enclosed in calcine or glass boxes, known to be highly resistant to thermal shock; therefore reducing the risk of immediate and complete release of the uranium. [4] Borosilicate nonetheless degrades over time and ion exchange becomes responsible for the gradual permanent sequestration of the material in between clay layers. Often the ion exchange capacity of the clay is not high enough and or not enough of it is present, allowing the uranium point source to migrate into underground water bodies.
Until the late 1990s, nuclear waste leaks remediation technology involved pumping contaminated groundwater out of the ground, treating it and re-pumping in. [5] Besides the significant costs associated with this method, it proved to be largely inefficient in cleaning groundwater in a sustainable fashion. This is partly due to uranium species that remain in the soil and could potentially redissolve in the aquifers over time. Several methods have since been explored to remediate radioactive leaks in underground water streams, two of which will be discussed below: one chemical and one biological. Some historical examples of these cases and their outcomes will also be presented.
Uranium plumes originating from nuclear waste migrates to nearby water bodies and dissolves in the liquid in the form of a uranyl divalent cation, UO2 2+ in the presence of oxygen becoming highly mobile. [5] In this remediation method, the contaminated plume is intercepted with a permeable barrier of reductive material - Zerovalent Iron Fe 0 (ZVI) being an excellent candidate for its reactivity, non-toxicity and cheapness - which reduces the Uranium to a solid form U(IV)O2 (s) that precipitates out of the solution. As shown in Figure 1, the barrier is designed not to restrict the flow of groundwater but rather selectively reduce the uranium species traveling through it. This method of in-situ remediation is known as reductive precipitation. The higher the difference in pe between the two redox reactions (iron oxidation and uranium reduction) the higher the rate of removal of the metals. [6] The slower the rate of reaction and or the higher the uranium content in the contaminated waters, the thicker the iron barrier required to remediate the situation. [7]
The thermodynamic favorability of such a reaction and the unreactive nature of the solid precipitate make this technology a very promising one. [8] Furthermore reduction of other species by zerovalent iron helps maintain the reducing conditions in the subsurface adding an additional layer of safety in the long-run. However, such an inherent change in subsurface conditions of the water may negatively affect the environment by causing side reactions that may disrupt organism ecosystems. Further studies are require to study such effects on a case by case basis. Historical example of the use of zerovalent iron barriers include the Bodo Canyon Disposal site in Colorado where several barriers were constructed to remove uranium amongst other dangerous species. The barriers were operated from August 1999 to June 2004 and the effluent concentration of uranium was reduced to less than 10 ppb. [9] Another case includes the Mecsek Ore Site in Hungary in 2003 where a zerovalent barrier helped reduce the concentration of uranium in the waters to less than 1% of the influent value. [9]
Another interesting method presented in the early 1990s is the microbial reduction of uranium (VI) to uranium (IV). This form of uranium is highly insoluble therefore making it a possible candidate for long term remediation immobilization of the radioactive compound. This method provides an inexpensive and non-intrusive solution to remediating radioactive contamination. [10] Microbial species such as the GS-15 strand oxidize acetate to carbon dioxide as part of their metabolism. In the presence of iron and in anaerobic conditions, the electrons go into the reduction of Fe (III) to Fe (II). [11] However, it was found that the reduction of uranium instead of iron can yield twice the amount of energy and therefore potentially be a more favorable reaction for the micro-organism. Side effects of this method may include: a) the reduction of Uranium into poorly soluble compounds, c) a resistance by the bacterial strain to the reduction of uranium, d) a reversal of environmental conditions to more oxidizing conditions, consequently re-solubilizing U(IV) into U(VI)
© Marc Khalaf. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] A. Andrews, "Radioactive Waste Streams: Waste Classification for Disposal," Congressional Research Services, RL32163, December 2006.
[2] J. A. Jacobs, "The Reuse and Recycling of Contaminated Soils,"Environmental Geosciences 5, 151 (1998).
[3] E. Davies, "Use of Uranium Drinking Water Standards Under 40 CFR 141 and 40 CFR 192 as Remediation Goals for Groundwater at CERCLA Sites," U.S. Environmental Protection Agency, 6 Nov 01.
[4] Y. Kagawa, N. Kurosawa, and T. Kishi, "Thermal Shock Resistance of SiC Fibre-Reinforced Borosilicate Glass and Lithium Aluminosilicate Matrix Composites," J. Mater. Sci. 28, 735 (1993).
[5] B. P. Dwyer and D. C. Marozas, "In Situ Remediation of Uranium Contaminated Groundwater," Sandia National Laboratory, SAND97-0337C, February 1997.
[6] K. J. Cantrell, D. I. Kaplan, and T. W. Wietsma, "Zero-Valent Iron For the In Situ Remediation of Selected Metals in Groundwater," J. Hazard. Mater. 42, 201 (1995).
[7] B. Gu et al., "Reductive Precipitation of Uranium(VI) by Zero-Valent Iron," Environ. Sci.Technol. 32, 3366 (1998).
[8] C. Noubactep, A. Schöner, and G. Meinrath,. (2006). Mechanism of Uranium Removal From the Aqueous Solution by Elemental Iron," J. Hazard. Mater. 132, 202 (2006).
[9] K. Bronstein, "Permeable Reactive Barriers for Inorganic and Radionuclide Contamination,," U.S. Environmental Protection Agency, August 2005.
[10] L. Newsome, K. Morris, and J. R. Lloyd, "The Biogeochemistry and Bioremediation of Uranium and Other Priority Radionuclides," Chem. Geol. 363, 164 (2014).
[11] D. R. Lovley et al., "Microbial Reduction of Uranium," Nature 350, 413 (1991).