 |
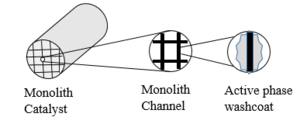
| Fig. 1: Catalyst coated on substrate. (Source: Y. Cao - after Thevenin et al. [1]) |
Emissions have been a growing concern around the globe. Catalytic combustion, operated at low temperature usually below 1400°C, is a promising way to reduce NOx, carbon monoxides and hydrocarbons emissions. [1] This has proven to be a greener alternative to conventional combustion for power generation. [1] Low temperature ignition of the fuel-air mixture is achieved with the help of metal catalysts, which need to have activity during the span of operation and be able to resist thermal shock during the combustion process. [1]
Catalysts increase reaction rates by lowering the activation energy barrier of chemical reactions. Catalysts used in low temperature combustion typically consists of a monolithic honeycomb substrate for mechanical stability, a washcoat layer to enhance surface area, and an active phase on which combustion reactions occur, as shown in Fig. 1. [1]
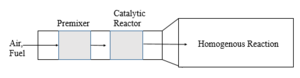 |
| Fig. 2: Flame holding mechanism. (Source: Y. Cao - after Smith et al. [2]) |
Common washcoat materials are alumina, hexaaluminates and perovskites, which not only provides high surface area for dispersion of active phase materials, but also have thermal expansion coefficients similar to the substrate to avoid cracks. [1] The active phase, made of metal oxides and/or platinum group metals, is tailored to achieve optimal catalytic activity. For instance, for jet and diesel fuel, a combination of precious metals including palladium, rhodium and platinum is used. [1]
For turbines with inlet temperatures below the limits of catalyst materials, a simple catalytic combustor has a premixer section through which air and fuel enter and proceed to the catalytic reactor. There are two mechanisms, as shown in Figs. 2 and 3, to sustain gas phase combustion: flameholding via backmixing of hot products, and catalyst-induced autoignition.[2] It is critical to maintain the temperature within the reactor below the maximum temperature allowed for catalysts. Factors such as mass transfer of reactants to the catalyst, chemical reaction rate on the catalyst surface, and channeling within the reactor can limit the degree of reaction. [2] When the flame temperature is high, two-stage catalytic combustion is used, as exemplified in Fig. 4. [2] The fuel-rich catalyst effluent in this case is mixed with cooling air prior to combustion to ensure the activity of the catalyst.
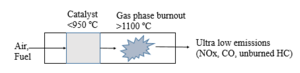 |
| Fig. 3: Backmixing mechanism. (Source: Y. Cao - after Smith et al. [2]) |
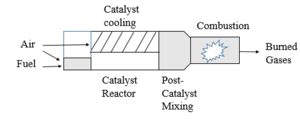 |
| Fig. 4: Two-stage catalytic combustion. (Source: Y. Cao - after Smith et al. [2]) |
Despite the gradual commercialization of catalytic combustion, there are still several challenges. The combustion process itself is highly exothermic with varying fuel/air ratios and fuel composition. The stability of catalyst during the entire operating process is thus a major determining factor of the effectiveness of catalytic combustion. In addition, the wetting of the catalyst surface caused by liquid fuel has to be avoided through robust revaporization technology. [2]
Catalytic combustion provides low NOx emissions compared to traditional combustion, and there has been huge progress in designing catalysts and combustion systems. It is crucial to maintain the stability of the catalyst throughout the combustion process for this technique to be feasible.
© Yi Cao. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] P. Thevenin, P. G. Menon, and S. G. Jaras, "Catalytic Total Oxidation of Methane. Part II. Catalytic Processes to Convert Methane: Partial or Total Oxidation," CATTECH, 7, 10 (2003).
[2] L. L. Smith et al., "Rich-Catalytic Lean-Burn Combustion for Low- Single NOx Gas Turbines." J. Eng. Gas Turb. Power 137, 27 (2005).