 |
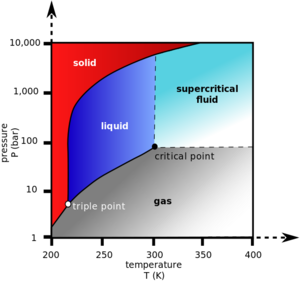
| Fig. 1: CO2 phase diagram illustrating supercritical region. (Source: Wikimedia Commons) |
Thermodynamic heat engine cycles are the underlying technology for the vast majority of electric power production methods, including nuclear, coal, natural gas, and solar thermal. Increasing the thermal efficiency of these cycles is a key factor in addressing growing global concerns regarding energy use and pollution resulting from burning of fossil fuels and storage of nuclear waste. In essence, a thermodynamic heat engine operates with a working fluid in four stages:
The working fluid is elevated to high pressure (either with pumps if a liquid or compressors if a gas). Work is done on the fluid during this process, which also increases its temperature.
The working fluid is heated at constant pressure to its highest temperature by a thermal energy source (burning coal, nuclear reactor, etc.). In a two-phase Rankine cycle, the working fluid is converted from a liquid to a gas. In a single-phase Brayton cycle, the working fluid is a gas which is simply heated further.
The working fluid is expanded to low pressure while rotating a turbine which produces mechanical work. This mechanical work may be used to spin an electric generator.
The working fluid is cooled at constant pressure to its initial state to allow the cycle to repeat.
While different mechanical plant and component designs, such as compressor intercooling (where a fluid is partially compressed and then cooled to require less work input for the remaining compression) and regeneration (where a portion of the cycle's rejected thermal energy is used to preheat the working fluid after compression) can be and are commonly used to increase the thermal efficiency, much attention has also been given to careful selection of the working fluid.
The working fluid typically runs through the cycle as a gas or changing phase between liquid and gas, depending on the cycle configuration. Each has its advantages. In a single-phase cycle dealing only with gas, there are no complications related to boiling or condensation, which requires careful design of the heat exchangers. In a two-phase cycle, the working fluid can be brought to high pressure as a liquid which requires less work input than the compression of a gas. At high enough pressure and temperature (above the fluid's critical pressure and temperature, respectively), there is no longer a definitive separation of gas and liquid phases and the fluid is said to be supercritical, as illustrated in Fig. 1. This allows for the mechanical simplicity of a single-phase fluid and the benefits of reduced compression work.
CO2 is of particular interest as a supercritical working fluid because its low critical temperature (31°C) and moderate critical pressure (7.38 MPa) allow for heat rejection to typical ambient conditions. In addition, the pressure is high enough that pressure losses due to piping are relatively low and modest enough that components do not require unrealistic reinforcement. While not conventionally used as a working fluid, the United States Department of Energy has begun sponsoring research to study supercritical CO2 turbomachinery design, as well as Na-CO2 heat exchangers for sodium-cooled Gen-IV nuclear reactors because of its favorable thermodynamic properties. [1]
The thermodynamic properties of supercritical CO2 allow it to match or exceed the performance of other high-efficiency cycles, like Brayton cycles using helium and transcritical (operating with some states as a supercritical fluid and some states as liquid or gas) steam Rankine cycles. However, while a helium Brayton cycle needs a high temperature around 850°C for thermal efficiencies in the mid-40%s, a supercritical CO2 cycle can reach them at substantially lower temperatures (below 600°C. [2]) Advanced, higher-temperature designs show promise to achieve thermal efficiencies of 55% or even higher. [3]
In addition to superior heat engine performance, the physical size of supercritical CO2 turbomachinery is relatively smaller than for other working fluids, and can involve many fewer expansion stages. A relative sizing illustration is given in Fig. 2. The supercritical CO2 power conversion unit is calculated to have a ~46% higher power density than that for helium. [2]
With state-of-the-art steam and gas turbine cycles typically running with roughly 40% thermal efficiency with relatively bulky turbomachinery, advances in supercritical CO2 cycle technology stand to make a substantial impact on the power generation market and the design of next-generation nuclear facilities. [4,5]
© Marc Dunham. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] A. Moisseytsev and J. J. Sienicki, "Investigation of Alternative Layouts for the Supercritical Carbon Dioxide Brayton Cycle for a Sodium-Cooled Fast Reactor," Nucl. Eng. Des. 239, 1362 (2009).
[2] V. Dostal, M. J. Driscoll, and Pl Hejzler, "A Supercritical Carbon Dioxide Cycle for Next Generation Nuclear Reactors," Massachusetts Institute of Technology, Advanced Nuclear Power Program, MIT-ANP-TR-100 10 Mar 04.
[3] S. A. Wright, "Mighty Mite," Mechanical Engineering Magazine, January 2012, p 40.
[4] G. J. Kolb, "An Evaluation of Possible Next-Generation High-Temperature Molten-Salt Power Towers," Sandia National Laboratories, SAND2011-9320, December 2011. [5] W. W. Bathie, Fundamentals of Gas Turbines, 2nd Ed. (Wiley, 1996), pp. 17-18.