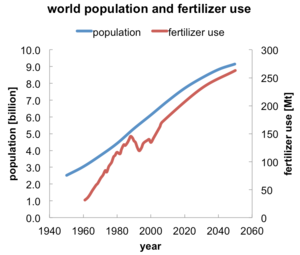
 |
| Fig. 1: World population and fertilizer consumption, with projections to 2050. [2] |
Synthetic fertilizers have sustained the population growth in the past century since they were introduced in 1914, and fertilizer production is expected to increase in the coming decades to feed an ever-growing world population (Fig. 1). [1,2] Fertilizer manufacture is an energy-intensive industry. It has been estimated that fertilizer production accounts for approximately 1.2% of the world's energy, of which about 93% is consumed by nitrogen-based fertilizers. [3,4] Unlike two other essential soil nutrients, phosphorous and potassium, nitrogen does not persist in the soil long after application, making frequent reapplication of nitrogen-based fertilizers necessary. [5]
The primary precursor of nitrogen-based fertilizers is ammonia (NH3), which is industrially synthesized from gaseous nitrogen (N2) and hydrogen (H2). [4] The process is known as the Haber-Bosch process:
This process was developed by Fritz Haber and Carl Bosch in the early 1900s using an iron catalyst discovered by Alwin Mittasch, and there has been no fundamental change to the process since it was initially developed. [6] Although the synthesis reaction is exothermic, high temperature and pressure conditions of 400-500 °C and 150-300 atm are required. [6-8] The reason lies in the counteractive responses of kinetics (overall reaction rate) and thermodynamics (conversion at equilibrium) to the change of temperature. The rate-limiting step of the reaction is the dissociation of adsorbed nitrogen molecules on the catalyst surface, known as dissociative nitrogen adsorption. [6,8,9] The use of a catalyst effectively lowers the activation barrier of this step but results in highly stable surface-bound nitrogen atoms. [6,8,9] A high reaction temperature is therefore needed to lift the nitrogen atoms out of the energy valley and keep the catalyst surface clean enough to maintain an industrially acceptable ammonia production rate. [7,9] However, since the reaction is exothermic, the yield of ammonia drops as the temperature increases, and an elevated pressure is needed to increase the equilibrium conversion, according to Le Châtelier's principle. [7,9] Thus, an ideal ammonia synthesis catalyst would be one that lowers the activation barrier of dissociative nitrogen adsorption yet does not bind too strongly with the nitrogen atoms. [9] In reality, such a catalyst is difficult to develop because the energy barrier of dissociative nitrogen adsorption and the surface-nitrogen binding strength are often strongly coupled. [9]
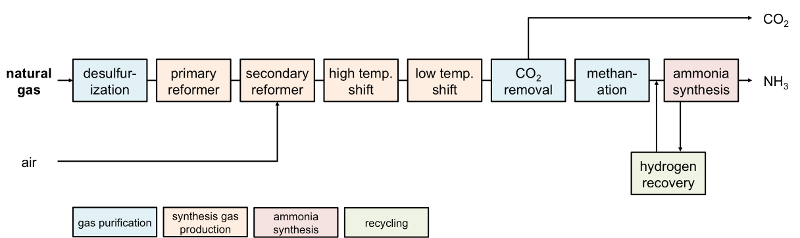 |
| Fig. 2: Conventional ammonia production process. [6] |
Yet, despite the need for a high reaction temperature and pressure, the Haber-Bosch process is only responsible for a small efficiency loss in the ammonia manufacture process. [10] In a typical ammonia production plant, natural gas provides the feedstock for hydrogen gas production via methane reforming (Fig. 2) and serves as a fuel for the plant. [3,11] Almost 70% of the loss of useful work ("exergy loss") is found in methane reforming and steam generation. [6] On closer inspection, natural gas feedstock used for hydrogen production accounts for more than half to even three-quarters of the total energy use of an ammonia production plant. [11] Indeed, in contrast to the Haber-Bosch process, drastic changes occurred over the years in the technology of producing a pure hydrogen-nitrogen mixture. [6]
Ammonia manufacture has evolved to become more energy-efficient since the early days of development. A modern ammonia production plant uses only 1.34 times the theoretical minimum energy (based on stoichiometry and lower heating value), a noticeable improvement from the classical Haber-Bosch process based on coke, which demands 3.38-4.31 times the theoretical energy requirement. [6] In addition, natural gas will likely remain the primary feedstock for industrial-scale hydrogen production in the near future. [12] Small improvements to ammonia manufacture may still be possible, but any change would be bound by the thermodynamic limits and have to compete with the high standards set by modern natural gas–fueled Haber-Bosch plants.
While revolutionizing the ammonia production process may seem a Herculean task, effort could be made to improve the efficiency and effectiveness of fertilizer application, thus saving the energy use associated with fertilizer production. Currently, there exists a regional imbalance in global fertilizer use. For example, large surpluses of nitrogen and phosphorous have been found in the North China Plain, degrading water and air quality through downstream and downwind losses. [13] In contrast, long-term insufficient fertilizer use in Sub-Saharan Africa has depleted soil fertility, resulting in inadequate food production and undernourishment. [13-15] In places with fertilizer overshoot, interventions focused on the environmental impacts are needed, which include better-targeted timing and placement of fertilizer application and modifications to livestock diets. [13] To replenish soil fertility in Sub-Saharan Africa, increasing fertilizer use through subsidies to smallholder farms will be necessary. [13] As an example, a scheme that allowed farmers to purchase fertilizers and improved maize seeds at 37% of the market price contributed to a tripling of maize yields in Malawi from 2005 to 2007. [15] Naturally available resources such as nitrogen-fixing tree fallows and indigenous rock phosphates are also useful supplements to synthetic fertilizers. [14,15] To bring about these changes, policy will arguably play a bigger role than science and technology.
© Mengyao Yuan. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] T. L. Roberts, "The Role of Fertilizer in Growing the World's Food," Better Crops 93, 12 (2009).
[2] N. Alexandratos and J. Bruinsma, "World Agriculture Towards 2030/2050: The 2012 Revision," Food and Agriculture Organization of the United Nations, ESA Working Paper No. 12-03, June 2012.
[3] "The Fertilizer Industry, World Food Supplies and the Environment," International Fertilizer Industry Association, December 1998.
[4] S. Wood and A. Cowie, "A Review of Greenhouse Gas Emission Factors for Fertiliser Production," IEA Bioenergy, June 2004.
[5] F. Tenkorang and J. Lowenberg-DeBoer, "Forecasting Long-Term Global Fertilizer Demand," Nutr. Cycl. Agroecosys. 83, 233 (2009).
[6] M. Appl, "Ammonia," in Ullmann's Encyclopedia of Industrial Chemistry, ed. by B. Elvers (Wiley-VCH, 2006).
[7] G. Marnellos and M. Stoukides, "Ammonia Synthesis at Atmospheric Pressure," Science 282, 98 (1998).
[8] G. A. Somorjai, Introduction to Surface Chemistry and Catalysis (Wiley, 2010), Ch. 7.
[9] T. H. Rod, A. Logadottir, and J. K. Nørskov, "Ammonia Synthesis at Low Temperatures," J. Chem. Phys. 112, 5343 (2000).
[10] R. Schlögl, "Catalytic Synthesis of Ammonia - A 'Never-Ending Story'?" Angew. Chem. Int. Ed. 42, 2004 (2003).
[11] L. Rafiqul et al., "Energy Efficiency Improvements in Ammonia Production - Perspectives and Uncertainties," Energy 30, 2487 (2005).
[12] T. Rostrup-Nielsen, "Manufacture of Hydrogen," Catalysis Today 106, 293 (2005).
[13] P. M. Vitousek et al., "Nutrient Imbalances in Agricultural Development," Science 324, 1519 (2009).
[14] P. A. Sanchez, "Soil Fertility and Hunger in Africa," Science 295, 2019 (2002).
[15] P. A. Sanchez, "Tripling Crop Yields in Tropical Africa," Nat. Geosci. 3, 299 (2010).