 |
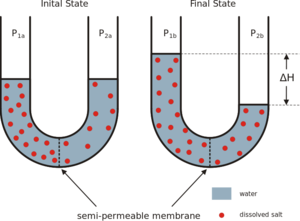
| Fig. 1: Through the process of forward osmosis, a chemical potential results from a concentration gradient of a dissolved species across a semi-permeable membrane. This provides the driving force for the development of a mechanical potential across the membrane from a difference in pressure. (Source: Wikimedia Commons) |
The current global population is approximately 7 billion people, and it is increasing faster than ever before. [1] Such a large population places increasing stress on the planet's natural resources. One of the most important resources is fresh water. Increasing urban consumption, use in burgeoning agriculture to feed growing citizenries, and maintenance of minimum levels of water to protect more and more natural ecosystems will continue to compete for a very limited supply of water. We need look no further than California to see the stress that drought can impose on society. Californians are feeling acutely the effects of what could be the worst drought in several hundred years. [2]
Luckily, there is a virtually infinite supply of water contained in our oceans. It is non-potable, however, because of it high salinity. The cost of desalinating ocean water remains too high to be competitive with more traditional collection methods. As with any resource, methods of procuring fresh water that today are prohibitively expensive will become economical as demand increases. Several interesting methods of desalination exist, but reverse osmosis appears to be the most promising process for the purification of seawater.
There is an absolute minimum amount of energy necessary to separate out the dissolved salt in seawater. The laws of thermodynamics dictate this minimum and no advances in membrane efficiency or upstream processing can drive the required energy expenditure below that level. The chemical equation for the mixing of sodium chloride and water is as follows
Solid sodium chloride has a free energy of formation (at 298 K) of -384.14 kJ/mol. Aqueous ions of sodium and chloride have free energies of formation (at 298 K) of -261.91 kJ/mol and -131.23 kJ/mol, respectively. [3] Dissolving sodium chloride in water therefore has a free energy of -9.00 kJ/mol. The free energy is less than zero, which agrees with the observation that dissolving salt is a spontaneous process. The reverse process, separating sodium chloride out from water, requires the input of energy equal in magnitude to the free energy of mixing. Given seawater's average salinity of 35 ppt = 0.60 mol/L, and converting units we can derive the commonly cited value of 1.5 kilowatt hours per cubic meter for the purification of sea water (it is important to note that the thermodynamic limit is dependent on temperature, local salinity, and final purified salinity). It should be stressed again that this is an incontrovertible minimum; no process will purify salt water with a smaller energetic cost than 1.5 kilowatt hours per cubic meter.
Consider two vessels that are separated by a special membrane. One vessel contains pure water. The other vessel contains a mixture of water and a salt solute. The membrane separating the two vessels allows water molecules across from one vessel to another. However, it does not allow the transport of salt molecules. We call this a semipermeable membrane. We observe the spontaneous movement of water from the vessel containing pure water into the vessel containing the dissolved salt. This process of water moving spontaneously across a semipermeable membrane is called osmosis. [4] It will continue until the increased pressure in the vessel with the salt halts the process. This equilibrium pressure is called the osmotic pressure. The theory of chemical potentials explains the water's movement. Just as a temperature gradient results in the flow of heat and a pressure gradient results in the expansion or contraction of matter, a gradient in the chemical potential results in the flux of chemical species to achieve constant concentrations. In this case, chemical potential and pressure are competing driving forces. Osmosis occurs until the two forces are balanced.
In reverse osmosis, an external pressure is imposed on the vessel containing the dissolved salt. As long as this pressure is greater than the system's osmotic pressure (defined by the thermodynamic variable like temperature and concentration), then water will flow out of the vessel containing the salt into the vessel containing pure water. Key here is that only water will flow, excluding salt. The amount of pure water will have then increased. By this process we can make pure water from our feed stream of salt water.
Reverse osmosis is a highly efficient process. It can purify seawater with an energetic cost not much greater than the minimum required by thermodynamics. In industrial applications, energy is used to power the pumps that pressurize the feed stream of seawater to the appropriate operating pressure. This one step accomplished the separation, so there is virtually no further loss of energy.
Reverse osmosis does not require much energy to purify water. It does, however, require more energy to purify water quickly. [5] The energy calculated above of 1.5 kilowatt hours per cubic meter tells us nothing about how quickly this process will occur. Another important, but entirely separate, metric is the volumetric flow rates out of a continuous flow reverse osmosis system. Generally, higher pressures will also increase flow rate. The type of separatory membrane is also very important. Weakened, corroded, or poisoned polymer membranes can decrease both purity and flow rate, requiring higher operating pressures and sometimes temperatures. [5] This increases the process's energetic demands and represents the area in which further research can add to the efficiency of industrial scale reverse osmosis desalination. We can never reduce the energetic budget below 1.5 kilowatt hours per cubic meter, but better composite materials can give us polymer membranes that are more robust and can tolerate process flow rates many times faster than today's operating parameters. The total operating cost cannot be greatly reduced today by decreasing energy demand, but cost can be driven down if the rate of production can be greatly increased.
&clopy; Kevin Hurlbutt. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] G. Gilbert, World Population: A Reference Handbook, 2nd Ed (ABC-CLIO, 2005).
[2] J. Medina, "With Dry Taps and Toilets, California Drought Turns Desperate," New York Times, 2 Oct 14.
[3] P. Atkins and J. de Paula, Physical Chemistry, 9th Ed. (W. H. Freeman, 2009).
[4] J. Kucera, Desalination: Water From Water (Wiley-Scrivner, 2014).
[5] W. S. Ho and K. K. Sirkar, Membrane Handbook (Springer, 1992).