 |
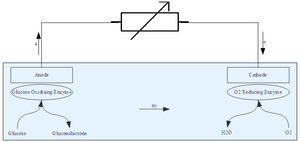
| Fig. 1: General glucose fuel cell operation. (Source: Wikimedia Commons) |
Glucose cells are devices that convert chemical energy from glucose fuel to electricity. They work by oxidizing glucose at one electrode (anode) and reducing oxidant at another (cathode). Charge flows through both sides of the cell as long as the fuel and oxidizing agent (usually oxygen) are supplied, providing electricity to the circuit. The efficiency of the fuel cell depends on its ability to catalyze the oxidation of glucose. To improve efficiency, enzymes or even living cells can be fixed on the electrode. Key factors in the design of glucose fuel cells include the choice of catalyzing materials, fixation of the enzymes, transfer of electrons, properties of the electrodes, and miniaturization of the fuel cell. [1] While currently limited to research use, glucose fuel cells hold promise as a renewable energy source and as a means for powering bionic implants. [2]
The most common reaction studied for glucose fuel cells is the oxidation of glucose to gluconolactone and the reduction of oxygen to water
This reaction provides 2 electrons per mole of glucose and a maximum reversible cell voltage of 1.3 V. In principle, the complete oxidation of glucose to carbon dioxide and water could provide 24 electrons per molecule, although in practice this reaction is difficult to achieve. Glucose fuel cells catalyze this reaction through one of three methods: (i) solid-state materials, (ii) enzymes, or (iii) living cells.
Solid-state fuel cells use the conductive surface of the electrode for catalyzing reactions. Due to their low specificity for glucose oxidation in the presence of oxygen, they are the least catalytically efficient, but are also the most simple and reliable. Important parameters for the electrode design include charge transfer efficiency and conductivity. The efficiency of solid-state catalysts can be further improved by increasing the surface area through roughening or grafting onto porous materials. [3] Enhanced efficiencies have been demonstrated through oxygen depletion designs that involve covering the anode with a porous cathode, such that most of the oxygen is reduced before reaching the anode. Glucose fuel cells based on platinum electrodes or activated carbon have been shown to generate on the order of 1-10 μW cm-2. [4]
Enzyme-based fuel cells have been extensively studied to increase the power output. Most work on glucose fuel cells have focused on the use of a single redox enzyme. A redox enzyme consists of the protein component and cofactors (non-protein components). The predominant enzyme used for glucose fuel cells is glucose oxidase (GOx) derived from Aspergillus niger due to its stability, selectivity, and availability. [5] Cofactors mediate the transfer of electrons from the enzyme to the electrode: common cofactors include flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NAD). Although electrons can be directly transferred to the electrode through tunneling, the transfer rate depends exponentially on the distance between the nearest donor and acceptor. Mediator cofactors are often introduced to shuttle electrons from the enzyme to the electrode in order to increase the current density. However, because the redox potentials of the mediated electron transfer now dictates the open-circuit potential of the cell, a central challenge in this method is to achieve the best compromise between the driving potential and the current. Effective use of enzymes for fuel cells requires a way to fix molecules near the electrode surface. These methods are important in most fuel cell applications because enzymes dissolved in solution at room temperature typically have an operational lifetime of only a few hours before they degrade. Strategies to immobilize enzymes involve mechanical compression, electrostatic binding, entrapment with polymer matrices, or covalent attachment to polymers. [6] Operational lifetimes of more than two years have been demonstrated using such strategies. [1]
When living cells are used to catalyze the oxidation of glucose, the device is referred to as a microbial fuel cell. Instead of transferring electrons to their characteristic acceptor, the transport is conducted over an anode. Using a graphite electrode and a microbes derived from sludge from a potato processing facility, power densities up to 3.6 W m-2 were demonstrated. [7] Organisms such as Rhodoferax ferrireducens have also been demonstrated to directly transfer electrons to graphite electrodes without mediators. [8] Crucially, the electrode materials must be biocompatible and chemically stable in the presence of the microbes. Most designs also require a separator between the anode and the cathode to prevent the electron acceptor (usually oxygen) from entering the anode chamber. The most commonly used separator material is Nafion, a commercially available cation exchange membrane. Microbes interestingly also produce their own nanowires ("pilli") to increase electron transfer, similar to roughing techniques used for solid-state fuel cells. [9]
The availability of glucose in natural body fluids makes glucose fuel cells a possible power source for biomedical implants. Glucose fuel cells for humans should optimize for oxygen and glucose concentration at the order of 10-4 M and 6 mM, respectively, in 150 mM chloride. [5] Due to challenges in introducing foreign microbes into the body, all density achieved by these fuel cells are quite low, necessitating large surface areas in order to power electronics. Glucose fuel cells consisting of enzymes mechanically compressed with carbon nanotubes were implanted in the abdominal cavity of rats, achieving a power density of 193.5 μW cm-2. [2]
© John Ho. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] S. D. Minteer et al., "New Materials for Biological Fuel Cells," Materials Today 15, No. 4, 166 (April 2012).
[2] A. Zebda et al., "Single Glucose Biofuel Cells Implanted in Rats Power Electronic Devices," Sci. Rep. 3, 1516 (2013).
[3] V. Oncescu and D. Erickson, "High Volumetric Power Density, Non-Enzymatic, Glucose Fuel Cells," Sci. Rep. 3, 1226 (2013).
[4] B. I. Rapoport, J. T. Kedzierski, and R. Sarpeshkar, "A Glucose Fuel Cell for Implantable Brain-Machine Interfaces," PLoS ONE 7, e38436 (2012).
[5] D. Leech, P. Kavanagh, and W. Schuhmann, "Enzymatic Fuel Cells: Recent Progress," Electrochim. Acta 84, 223 (2012).
[6] A. Zebda et al., "Mediatorless High-Power Glucose Biofuelcells Based on Compressed Carbon Nanotube-Enzyme Electrodes," Nat. Commun. 2, 370 (2011).
[7] K. Rabaey et al., "A Microbial Fuel Cell Capable of Converting Glucose to Electricity at High Rate and Efficiency," Biotechnol. Lett. 25, 1531 (2003).
[8] S. K. Chaudhuri and D. R. Lovley, "Electricity Generation By Direct Oxidation of Glucose in Mediatorless Microbial Fuel Cells," Nat. Biotechnol. 21, 1229 (2003).
[9] B. E. Logan et al., "Microbial Fuel Cells: Methodology and Technology," Environ. Sci. Technol. 40, 5181 (2006).