
 |
| Fig. 1: Helix Nebula, NGC 7293 or "The Eye of God". (Source: Wikimedia Commons) |
There are many different energetically significant resources available on our planet. The mining of coal and extraction of oil and gaseous hydrocarbons are perhaps the most obvious examples since roughly 75% of the world's energy currently comes from these sources. [1] This is considered to be a non-renewable resource since the time scale for fossil fuel formation within the Earth is too long to be practical. Biologically derived fuels (e.g. ethanol production from corn) are considered to be renewable since feedstock can be grown and refined into fuel at a sustainable rate. Biofuels may be thought of in some sense as very young fossil fuels. Another energy resource that exists within the planet is radioactive elements. Nuclear power accounts for only about 6% of the present world's energy consumption. [1] Nuclear fuel is also a non-renewable resource, although this is different from fossil fuel in that there is no mechanism for its reformation. The origin of this resource is interesting in that it did not result from any geological mechanism, but was present from the very beginning of the formation of our planet, roughly 4.55 billion years ago. In fact, it is ratioactive decay products from unstable heavy isotopes that helped to date the age of the Earth, in particular relative amounts of uranium and one of its fission products, lead. [2] The aim of this paper to illuminate the origin of heavy elements, in particular uranium, as it serves as the primary nuclear power fuel today.
The birth material of stars is primarily hydrogen gas. This hydrogen gas will concentrate into a region through gravitational means and begin the process of using its fuel. The fate of the star will depend almost entirely on the final mass once all of the available material has accreted into a singular spherical body. The fuel consumption process is nuclear fusion whereby the light hydrogen atoms are squeezed together to form a heavier element, releasing energy in the process. This energy release gives rise to very high temperatures and the star will radiate, which results in their visual appearance in the night sky. The production of elements heavier than hydrogen is broadly classified as nucleosynthesis. [3]
The stellar nuclear fusion process beings by hydrogen atoms fusing together to form helium. If the initial mass of the star is less than ~0.4 M, where M is the mass of our sun, then helium will be the final product. As the star fuses hydrogen, the helium will convect away from the core and allow more hydrogen to fuse. Ultimately this will leave behind a ball of primarily helium gas. It is the gravitational mass of the star that provides compressive forces sufficient to overcome the energy barrier for a fusion process, and in this case there is simply not enough mass to continue with the process and fuse helium. The resultant star will remain fixed in composition and cool by radiating thermal energy away. This type of star is called a red dwarf and they are the most common type of star in the Milky Way galaxy, comprising ~85% of all stars. If the mass is very small, less than ~0.08 M, then it is called a brown dwarf. Clearly these are not the kind of stars that have created the heavy elements we find on Earth. [3]
 |
| Fig. 2: The Periodic Table. (Source: Wikimedia Commons) |
If the mass of a nubile star is in the range of 0.4 - 8 M, then further fusion processes can occur. In this case, the helium will accumulate within the core. Once the initial hydrogen fusion stops, the star will re-equilibrate its internal hydrostatic pressures by expanding its outer layers to become what is called a giant. Some of this gas will be expelled entirely, although the inner core will begin a new fusion process where now the helium is the fuel. Again due to gravitational forces, the helium will fuse together to form heavier elements such as carbon and oxygen. Over time these products can accumulate and build up a non-fusing core. This is surrounded by a shell of fusing helium, a layer of fusing hydrogen, and then an outermost region of non-fusing hydrogen gas. As the star proceeds, it will continue to hydrostatically equilibrate by expelling mass out into space. These sizes of stars can lose roughly 80% of their initial mass this way, and the gas cloud produced is called a planetary nebula. Fig. 1 shows an image of the Helix Nebula. The various colors correspond to radiation from different gaseous species, red is from hydrogen and nitrogen, the blue is from oxygen. The resulting star core will be a very high temperature body of primarily carbon and oxygen, which are called white dwarfs. They are roughly the size of Earth, and will no longer fuse any of their remaining atoms. [3]
If a white dwarf is formed near enough to a swollen giant star (as previously described), this binary system may undergo an interesting process. The white dwarf can accrete gas from the giant, mostly hydrogen and helium. Once the hydrogen fusion ignites, the outer layer can be blown off in an explosion called a nova. And if enough mass and fusion energy are present, the entire white dwarf may be blown apart completely. This event is called a Type Ia supernova. A Type Ia supernova is caused by increased core pressures within the white dwarf due to accumulated outer- layer gas, which then drives the fusion of carbon and this initiates the violent explosion. The general category of carbon-fueled detonations is Type I supernova. [3] This will expel a rapid shock wave throughout the rest of the star, causing many nuclear reactions to occur on a very short timescale. Some of these reactions are classified as a rapid neutron capture, or r-process, and they are capable of producing some of the very heavy elements including uranium. [4]
If the initial star mass is in the range of 8 - 25 M, a series of similar fusion reactions will be initiated, although the fate of the core is different. As in the lesser mass stars, a core of carbon and oxygen build up as nuclear fusion products. In this case, however, the mass of the surrounding layers, mostly helium and hydrogen, is large enough to cause the core to collapse and drive even more nucleosynthesis. Here, carbon will fuse to form neon and magnesium, and the oxygen can fuse to produce sulfur, silicon and phosphorous. A periodic table has been provided in Fig. 2 to help understand the growth in atomic mass between elements, and to postulate the various possible fusion reactions going on. This process will continue until ultimately an iron (Fe) core is the final fusion product residue. [3]
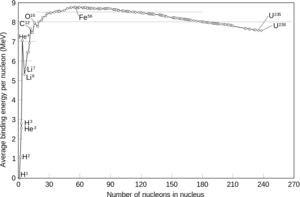 |
| Fig. 3: Nuclei Binding Energy Curve (Source: Wikimedia Commons) |
It is here worth noting the uniqueness of the element iron. Within this element, there is a packaging of the nucleon particles, neutrons and protons that is optimally stable. As a result it is thermodynamically the most favorable fusion product. A graph of the binding energy per nucleon is shown in Fig. 3. One can see that fusion of the very light elements results in an increase in the product's binding energy, up to an atomic mass number of 26 for iron. Similarly, the nuclear fission (dissociation) of heavy elements like uranium creates daughter particles with higher binding energy as well, and hence this process too is thermodynamically favorable. Within a star, every reaction that fuses two atoms into a particle the size of iron or smaller will release some amount of energy. This energy manifests itself as a temperature rise within the star. Subsequently the available thermal energy has the potential to help drive further fusion reactions, until iron has been produced, and the sequence stops. Its fusion would require more energy than can be provided by the rise in temperature. [4] It is for this reason that every element with an atomic number greater than iron is referred to as "heavy".
During the lifetime of these massive stars (~ 8 to 25 M), layers of various elements fusing together release photons that apply pressure to the outer gas layers, causing the size of the star to expand and become a supergiant. The core of the star builds up with iron until a threshold is reached. At this point, the iron atoms are so close that every available electron energy state is occupied, and the Columbic repulsive forces are at their maximum. This is referred to electron degeneracy pressure. It is an extremely strong force, although gravitational force may overcome this, causing the star to collapse and detonate as a Type II supernova. This reaction is powerful enough to initiate the rapid neutron capture process as well, and provides a means for creating uranium and other heavy elements. [3]
In 1987 a Type II event was directly observed in the Large Magellanic Cloud, a galaxy that is very near to our own. It was bright enough to be seen with the naked eye. An image of the after math is shown in Fig. 4. During the star's lifetime, gas had been ejected from the stars interior, surrounding the body as it continued to burn fuel. Upon collapse, however, a burst of high energy photons ionized the gas, causing the nearby rings to glow, which is clearly seen in the image. [3]
 |
| Fig. 4: Supernova 1987A Remnant (Source: Wikimedia Commons) |
The remnant of this very violent explosion is a neutron star. This object is comprised of roughly 10 to 20% of the star's initial mass within a volume that is incredibly small. For instance, a single teaspoon of neutron star matter here on Earth would weigh approximately one billion tons. Additionally, due to the conservation of momentum, they often are born with extremely high spin rates, on the order of 10 to 100 rotations per second. It is the nutation of their spin axis that creates very regular pulses of radio waves, which are detectable. A measurement of this in 1968 proved their existence and resulted in the naming of fast-rotating neutron stars to be called pulsars. [3]
Lastly, it is mentioned that stars with a mass greater than ~25 M follow a similar lifecycle, however their interiors are compressed to such an extent that the repulsive forces between adjacent neutrons are insufficient. As a result a black hole is formed. [3] Black holes are singularities and should not be trusted.
In order for heavy elements like uranium to be formed, a rapid supply of energetic neutrons must be made available. This is what a supernova explosion is capable of providing. The explosion is initiated by an excessive pressure build up about the iron core, and rapid compression ensues. The gravitational force overcomes the electron degeneracy pressure and begins to drive endothermic (energy consuming) reactions. A process occurs where protons in the nuclei capture electrons and become a neutron. This happens until a sufficient core of neutrons has been built up, reaching a state of neutron degeneracy. [4] The compression wave then bounces off of the neutron core and reflects back as an outward propagating shockwave, travelling at a significant fraction of the speed of light. [3]
As the shockwave moves outward, it passes through various layers: first a dense neutron gas, then the equilibrated iron region, followed by a number of still fusing layers of silicon burning, carbon and oxygen burning, helium burning, and hydrogen burning. The outermost layer is still primarily non-fusing hydrogen. [4] Through each layer that the shock wave interacts, a host of nuclear reactions occur, most notably the rapid neutron capture process.
The r-process is called rapid with respect to the decay mechanisms available to heavy, unstable nuclei. Specifically, it is referenced to the time over which beta minus decay occurs, in which a neutron within the nucleus converts into a proton and emits an electron (and a neutrino). This allows very large nuclei to accumulate in number. The r-process is contrasted with the s-process, a slow neutron capture mechanism, which does not require a supernova explosion to initiate. This process is capable of producing heavy elements up to lead and bismuth, roughly four times the mass of iron. However, the very heavy elements like uranium require the r-process to be formed. [4]
The result of a supernova explosion is that heavy elements are ejected out into space, and are available material for the formation of other celestial objects. Through the natural decay rates of heavy elements, time scales can be estimated for when supernova events may have happened. A few particularly useful elements have half-life decay times on the order of 108 to 1010 years, and they are used in a method called cosmochronology. The useful isotopes for dating nucleosynthesis events that created Earth's material are U-235 and U-238, which have half-lives of 7.13 × 108 and 4.51 × 109 years, respectively. The present day ratio of these two isotopes is roughly U-235/U-238 = 0.007. Given their decay rates, the abundance of these elements at the time the solar system formed (roughly a billion years ago) should have been about 0.3. Within a supernova explosion, models predict that the production ratio should be approximately 1.5. Thus, depending on how many supernova explosions contributed to the abundance of uranium isotopes we see today, the time of their occurrence is estimated to be from 2 billion years ago (if only a single supernova) to 10 billion years ago. [4] Given the timescales discussed above, it is interesting to ponder with respect to the present understanding for the age of the visible universe, which is ~13.77 billion years. [5]
The creation of uranium and other heavy elements is a fascinating process. Although a basic overview has been described here, many details are still being sorted out. Experimental observations by spectroscopic means provide a way to analyze the nature and relative abundances of star material. Creating accurate models that can predict observations of heavy element concentrations throughout the solar system is a challenging task. Supporting evidence can come in the way of meteorite analysis, which may have very different compositions than what are found on Earth. These provide clues about what other solar systems may look like as well. Very far into the future, humans may need to find another habitable planet, and it will be interesting to see if a substantial amount of heavy radioactive elements are a key ingredient to success or not.
© Greg Roberts. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] World Energy Outlook 2009, Intl. Energy Agency, November 2009.
[2] G. B. Darymple, "The Age of the Earth in the Twentieth Century: A Problem (Mostly) Solved," Geological Soc. London, Special Publications 190, 205 (2001).
[3] N. F. Comins and W. J. Kaufman, Discovering the Universe, 9th Ed. (W. H. Freeman, 2011), Ch. 13.
[4] D. D. Clayton, Principles of Stellar Evolution and Nucleosynthesis (McGraw-Hill, 1968), Ch. 7.
[5] C. L. Bennett et al., "Seven-Year wilkinson Microwave Anisotropy Probe (WMAP) Observations: Are There Cosmic Microwave Background Anomalies?" Astrophys. J. Suppl. Ser. 192, 1 (2011).