 |
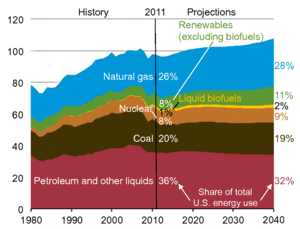
| Fig. 1: U.S. primary energy consumption by fuel, 1980-2040 (unit: quadrillion Btu per year). [1] (Courtesy of the U.S. Department of Energy.) |
Approximately 25% of the United States' energy production is currently derived from natural gas. [1] Natural gas will continue to play a significant role in meeting the energy demands in the coming decades (see Fig. 1) due to its abundance and environmental soundness. [1] For example, natural gas reserves are estimated to be 187.3 trillion cubic meters, which is sufficient to meet 55.7 years of global energy production at the moment. [2] Burning natural gas also produces more heat per mass (15.21 kWh per kg) but less carbon dioxide per unit of heat released (0.55 kg per kWh) than any other hydrocarbon fuels because natural gas consists of mostly methane (CH4), the simplest hydrocarbon. [3,4] In addition, natural gas has multiple applications across the chemical industry as methane can be converted to synthetic gas, a raw material for the manufacture of various chemical compounds.
Although natural gas is a decent source of energy, the production and the use of natural gas are strongly limited by the difficulties in transporting natural gas. Due to its low energy density by volume (10.75 kWh per cubic meter), natural gas needs to be transported as a compressed gas (10 - 100 atm), which is energy intensive and expensive. [1] As a consequence, natural gas obtained as a byproduct in oil drilling is often vented or flared (see Fig. 2) merely due to economic reasons. According to National Oceanic and Atmospheric Administration (NOAA), the global flaring of petroleum-associated gas accounts for up to 140 - 170 billion cubic meters of natural gas, which corresponds to approximately 25% of the annual U.S. natural gas consumption. [5] Thus, we are in need of a catalyst technology that can convert natural gas to a liquid in order to increase the extent and efficiency of natural gas utilization.
 |
| Fig. 2: North Dakota flaring of natural gas out of the Bakken Formation. (Source: Wikimedia Commons.) |
Developing a direct route for conversion of methane to methanol can provide the foundation for efficient utilization of natural gas. Methanol (CH3OH) is an energy-dense liquid that can be transported easily with existing infrastructure. In addition, it is a versatile molecule as it can be used for fuel cells; blended with gasoline; converted to gasoline or dimethyl ether, which is a component of diesel fuel; and converted to ethylene and propylene, which are precursors to a wide range of chemicals. Given the chemical properties and versatility of methanol as well as the abundance of natural gas, the direct conversion of methane to methanol is indeed a dream process that we need to achieve.
The catalytic conversion of methane to methanol has been under investigation for almost a century, but a commercial process has yet to be developed. It has been difficult to find suitable catalyst materials that can convert methane to methanol with an economic yield of 10% or above. [6] In most experiments with solid catalysts, selectivities to methanol fell rapidly as methane conversions exceeded 59%. [6] This can be explained by thermodynamics that complete oxidation of methane to carbon dioxide (ΔH = -877 kJ/mol) is highly favored over partial oxidation of methane to methanol (ΔH = -200 kJ/mol). [7]
A noticeable progress, however, has been made in the field of molecular catalysis by Periana et al., who demonstrated the selective conversion of methane to methanol at temperatures around 473 K over platinum bipyrimidine complexes. [8] According to their experiment, 81% selectivity to methyl bisulfate, a methanol derivative, was reached at methane conversion of 90% in concentrated sulfuric acid. Although these results are promising, commercial applications are hampered by difficult separation and recycling of the molecular catalyst. [9] A solid catalyst, with its advantages of easy separation and recyclability, is strongly desired to facilitate the implementation of the methane-to-methanol technology.
To develop a solid catalyst based on the work by Periana et al., Palkovits and coworkers have taken the approach of using a polymer framework as a solid matrix for catalytic oxidation of methane to methanol via methyl bisulfate in concentrated sulfuric acid. [9] A thermally stable polymer was treated with a platinum precursor to produce platinum coordination sites that are similar to those of platinum bipyrimidine complexes. As a result, Palkovits and coworkers successfully synthesized solid catalysts that showed catalytic activities and selectivities comparable to those of platinum bipyrimidine complexes. Despite these promising results, however, one-step conversion of methane to methanol, i.e., without methyl bisulfate as an intermediate, is still desired. [10]
Developing a technology that can convert methane to methanol is necessary to conveniently transport natural gas from its production regions to consumption regions. Although many achievements have been made in catalyst development, we need to continue our efforts to find alternative materials that can actively and selectively catalyze the partial oxidation of methane to methanol in order to allow efficient utilization of natural gas.
© Jong Suk Yoo. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] "Annual Energy Outlook 2013," U.S. Energy Information Administration, DOE/EIA-0383(2013), April 2013.
[2] "BP Statistical Review of World Energy 2013," British Petroleum, June 2013.
[3] "An Assessment of the Greenhouse Gas Consequences the Proposed Batchelor Magnesium Project," Northern Territory, Australia, 20 Feb 12.
[4] "Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2010," U.S. Environmental Protection Agency, EPA 430-R- 12-001, April 2012.
[5] C. D. Elvidge et al., "A Fifteen Year Record of Global Natural Gas Flaring Derived from Satellite Data," Energies 2, 595 (2009).
[6] P. S. Casey et al., "Selective Oxidation of Methane to Methanol at High Pressures," Ind. Eng. Chem. Res. 33, 1120 (1994).
[7] M. J. Brown and N. D. Parkyns, "Progress in the Partial Oxidation of Methane to Methanol and Formaldehyde," Catal. Today 8, 305 (1991).
[8] R. A. Periana et al., "Platinum Catalysts for the High-Yield Oxidation of Methane to a Methanol Derivative," Science 280, 560 (1998).
[9] R. Palkovits et al., "Solid Catalysts for the Selective Low-Temperature Oxidation of Methane to Methanol," Angew. Chem. Int. Ed. 48, 6909 (2009).
[10] C. Hammond et al., "Direct Catalytic Conversion of Methane to Methanol in an Aqueous Medium By Using Copper-Promoted Fe-ZSM-5," Angew. Chem. Int. Ed. 51, 5129 (2012).