Over the last century, polymers have become prevalent in many commercial products that humans use in their daily lives. The ability to manufacture polymers cheaply and efficiently has played a significant role in the advancement of plastics and their replacement of many metal and ceramic products. While polymers are simply long chains composed of repeating monomer units, the ability to tailor the properties of polymers by give them a variety of engineering uses and scientific uses. [1] One of the significant advantages to using polymers is their relatively low melting point compared to ceramics and metals, making them much more easily shaped into useful technology. However, the exact properties that lead to the useful properties of this polymer are controlled on the molecular level, in which the monomer units are assembled. Reactions that combine these monomer units are commonly referred to as polymerization reactions, and many different mechanisms are used to link them into polymers. [2] One of the more common mechanisms used in polymer chemistry is radical chain polymerization (also sometimes referred to as a living polymerization).
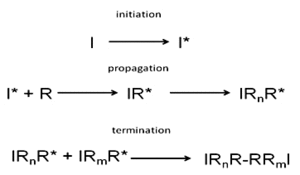
Radical chain polymerization occurs in three steps, as shown in Fig. 1. The first stage of the reaction is initiation, in which a free radical is created, typically by breaking the O-O bond in a peroxide like benzoyl peroxide. [2] The free radical is then transferred to the monomer, forming an active center that can attack other monomers. This step is called propagation, in which the free radical is propagated down the polymer chain. The final step is termination, in which two molecules containing free radicals react and form the final product. While this reaction is fairly simple, purifying the product of the initiator is one of the main drawbacks of using chemical initiators. Furthermore, the purity of the initiators themselves also becomes a concern for both purification and controlling the reaction products. [3] In order to overcome the limitations of free radical polymerization, researchers have studied using gamma radiation to induce polymerization reactions. Polymerization using γ-rays proceeds via the same mechanism as the free radical initiation mechanism described previously, but without the use of chemical initiators. [3, 4] By using the γ-rays to initiate polymerization reactions, the purification process is dramatically simplified. Furthermore, the reaction rates can be more precisely controlled and manipulated in-situ by manipulating the radiation dose. [3-5]
Recently, some polymerization reactions have been demonstrated using γ-ray irradiation. One such reaction is the polymerization of methacrylates for metal ion absorption. These polymers can be effective absorbers of toxic metal ions, such as Cr6+ and Pb2+ from aqueous solutions, making them useful for water purification. The mechanism for the reaction is shown in Fig. 2. The reaction is carried out at a dose of 20 kGy at 0.5 kGy/hr using a Co-60 radiation source, which was previously shown to be the optimal conditions for the reaction. [6] Varying the radiation dose changes the completeness of the polymerization reaction. It was found that iron polymethacrylates were significantly more effective compared to standard chromium sorbents. [7] Other polymerization reactions have also been explored and used in applications such as polymer cross-linking, and extensively as a means to graft specific monomers onto polymer chains without the use of chemical inhibitors. [5]
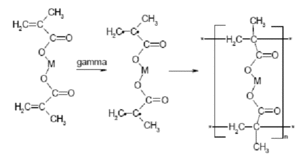 |
| Fig. 2: Reaction mechanism for polymerization of metal methacrylates. |
Polymerization via γ-rays offers an interesting new method of controlling polymerization reactions. While some specific examples of γ-ray induced polymerization were shown here, its application to biomedicine and functional materials has also been studied. [8] It has been used to functionalize the surfaces of polymers with different chemical groups, as well as to graft new polymer chains onto existing functional materials to alter their properties. [9] γ-ray induced polymerization is a relatively old, yet not widely used technique in radiation chemistry. As a relatively unknown field of chemistry, radiation chemistry has also provided valuable insight into reaction mechanisms and chemical kinetics. [4] As radiation source technology adapts, becomes more widely available, and more fields begin to utilize the use of high energy radiation, new and exciting uses for this technology may emerge.
© Ben Weil. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] M. De Graef and M. E. McHenry, Structure of Materials (Cambridge U. Press, 2007).
[2] G. M. Loudon, Organic Chemistry, 4th Ed. (Oxford U. Press, 2002).
[3] "Advances in Radiation Chemistry of Polymers", International Atomic Energy Agency, IAEA-TECDOC-1420, November 2004.
[4] V. Markovic, "Radiation Chemistry: Little Known Branch of Science," Intl. Atomic Energy Agency Bull. 31, No. 1, 20 (1989).
[5] R. L. Clough, "High-Energy Radiation and Polymers: A Review of Commercial Processes and Emerging Applications," Nucl. Inst. Meth. Phys. Res. B 185, 8 (2001).
[6] A. Galvan-Sánchez et al., "Determination of the Crystallinity Index of Iron Polymethacrylate," J. Appl. Polymer Sci. 74, 995 (1999).
[7] B. Bilyeu, C. Barrera-Díaz and F. Ureña-Nuñez, "Gamma Radiation-Polymerized Metal Methacrylates for Adsorption of Metal Ions from Wastewater, in New Membranes and Advanced Materials for Wastewater Treatment, ed. by A. Mueller, B. Guieysse and A. Sarkar (American Chemical Society, 2010), pp. 71.
[8] M. Fujitsuka, and T. Majima, "Recent Approach in Radiation Chemistry Toward Material and Biological Science," J. Phys. Chem. Lett. 2, 2965 (2011).
[9] M. Carenza, "Recent Achievements in the Use of Radiation Polymerization and Grafting for Biomedical Applications," Radiat. Phys. Chem. 39, 485 (1992).