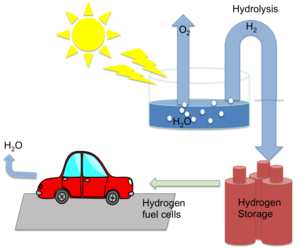
 |
| Fig. 1: The transformation of solar energy into chemical energy stored in the form of hydrogen, through photoelectrochemical water splitting |
Development of new energy sources is a major need for the 21st century. Hydrogen is one of the ideal fuels sources for the future since Hydrogen fuel can be produced from clean and renewable energy sources and, thus, its life cycle is clean and renewable especially if it can be generated inexpensively from an abundant raw material such as water. The other main reason to consider Hydrogen as an alternative fuel is because it is carbon-free, facilitates use of more efficient power generation systems (e.g., fuel cells), and can be used to chemically reduce carbon oxides (CO, CO2) to chemical fuels.
Solar-to-chemical energy conversion of water to H2 is especially attractive given the abundance of water and of the "free" energy available in sunlight. However, presently, 95% hydrogen is mainly derived from fossil fuels and renewable energy contributes only about 5% of the commercial hydrogen production primarily via water electrolysis. Cost of renewable hydrogen production is still high. This is the main reason why it not popular yet. Photovoltaic water electrolysis can become more competitive as the cost continues to decrease with the technology advancement. [1]
Alternatively, photocatalytic water-splitting using TiO2 for hydrogen production offers a promising way for clean, low-cost and environmentally friendly production of hydrogen by solar energy. Early work of TiO2 photoelectrochemical hydrogen production was reported by Fujishima and Honda where titania was used as an anode photocatalyst. [1]
The electronic structure of a semiconductor plays a key role in semiconductor photocatalysis. Unlike a conductor, a semiconductor consists of valence band and conduction band. Energy difference between these two levels is said to be the band gap (Eg). When a semiconductor is excited by a photon with energy equal to or higher than the semiconductor band gap energy level, electrons receive enough energy from the photon and are thus excited from valence band to conduction band. It only happens if the energy gain is higher than the band gap energy level of semiconductor. As a result there will be photo-generated electrons and holes that can recombine in bulk or on surface of the semiconductor within a very short time and release energy in the form of heat or photons.
Electrons and holes that reach to the surface of the semiconductor without recombination can, respectively, reduce and oxidize the reactants adsorbed by the semiconductor. The reduction and oxidation reactions are the basic mechanisms of photocatalytic hydrogen production and photocatalytic water/air sanitization, respectively. Requirement condition for hydrogen production is that the conduction level should be more negative than hydrogen production level (EH2/H2O) while the valence should be more positive than water oxidation level (EO2/H2O ) for efficient oxygen production from water by photocatalysis. The photocatalytic hydrogen production by TiO2 is shown in Fig. 2.
 |
| Fig. 2: Mechanism of TiO2 photocatalytic water-splitting for hydrogen production. |
In theory, all types of semiconductors that satisfy the above-mentioned requirements can be used as photocatalysts for hydrogen production. However, most of the semiconductors, such as CdS and SiC, that cause photocorrosion, are not suitable for water-splitting. Among all semiconductors, having strong catalytic activity, high chemical stability and long lifetime of electron/hole pairs of TiO2 makes is the most widely used photocatalyst. [2] But while TiO2 is an attractive material because it is inexpensive and a well known catalyst for water dissociation, there are also several other parameters that need to considered. The energy conversion efficiency from solar to hydrogen by TiO2 photocatalytic water-splitting is still low, mainly due to the following reasons:
Recombination of photo-generated electron/hole pairs: conduction band electrons can recombine with valence band holes very quickly and release energy in the form of unproductive heat or photons.
Fast backward reaction: Decomposition of water into hydrogen and oxygen is an energy increasing process, thus backward reaction (recombination of hydrogen and oxygen into water) easily proceeds. [2]
One other major drawbacks that limits is practicality is that it has a high band gap of 3 eV, which absorbs photons in the UV range, utilizing only 5% of solar radiation at most. In order to induce a better charge separation within a semiconductor film, a wide variety of approaches have been considered. One such approach is to sequentially couple different types of semiconductor materials where it becomes possible to promote charge separation by accumulating charge carriers (e-, h+) in two or more semiconductor layers. [3] O’Reagan and Grätzel developed a dye-sensitized solar cell, where the electrolyte continuously oxidizes and reduces the dye molecules leaving behind no net change and a dye absorbing light in the visible region resulting in a photoexcited electron being rapidly injected into the conduction band of TiO2. [4] Another approach involves the deposition of noble metal nanoclusters in order to maximize the efficiency of photocatalytic water splitting reactions. [5,6] The role of the noble metal is to store photogenerated electrons and prevent them from recombining with holes in order to promote interfacial charge transfer within these composites. [7]
Hydrogen is one alternative clean fuel for micro-scale devices when generated by solar irradiation through water splitting. While TiO2 is an attractive material because it is inexpensive and a well known catalyst for water dissociation, there are several major drawbacks that limits its practicality. A number of modification techniques and chemical additives have been developed in recent years to improve photocatalytic activity of TiO2 under visible light irradiation. Applications include the promising photocatalytic watersplitting capability for hydrogen production. It is anticipated that the low cost, environmentally friendly photocatalytic water-splitting for hydrogen production will play an important role in the hydrogen production and contribute much to the coming hydrogen economy.
© Rahim Esfandyarpour. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] A. Fujishima and K. Honda, "Electrochemical Photolysis of Water at a Semiconductor Electrode," Nature 238, 37 (1972).
[2] M. Ni et al., "A Review and Recent Developments in Photocatalytic Water-Splitting Using TiO2 for Hydrogen Production," Renewable and Sustainable Energy Reviews 11, 401 (2007).
[3] S. Hotchandani and P. V. Kamat, "Charge-Transfer Processes in Coupled Semiconductor Systems. Photochemistry and Photoelectrochemistry of the Colloidal Cadmium Sulfide-Zinc Sulfide System," J. Phys. Chem. 96, 6834 (1992).
[4] B. O'Reagan and M. Gräzel, " A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films," Nature 353, 737 (1991).
[5] A. J. Bard, "Design of Semiconductor Photoelectrochemical Systems for Solar Energy Conversion," J. Phys. Chem. 86, 172 (1982).
[6] R. Baba et al., "Investigation of the Mechanism of Hydrogen Evolution During Photocatalytic Water Decomposition on Metal-Loaded Semiconductor Powders," J. Phys. Chem. 89, 1902 (1985).
[7] N. Chandrasekhran and P. V. Kamat, "Improving the Photoelectrochemical Performance of Nanostructured TiO2 Films by Adsorption of Gold Nanoparticles," J. Phys. Chem. B 104, 10851 (2000).