 |
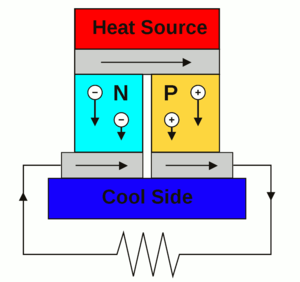
| Fig. 1: A thermoelectric power generator. The same circuit can be used as a solid-state cooler. (Source: Wikimedia Commons) |
In 1820, the Estonian-German physicist Thomas Johann Seebeck discovered that a magnetic field is present near a closed loop of two different metals or semiconductors which are held at different temperatures. [1] Hans Christian Oersted had previously shown that an electric current will give rise to a magnetic field, so Seebeck's discovery came to be known as thermoelectricity. [1] In 1834, the French physicist Jean Charles Athanase Peltier discovered the inverse effect, that running current through a junction of different metals will produce a temperature difference between them. [2] This effect has been used to cool electronics for over five decades, and has also historically been used to power spacecraft from the heat generated by the decay of radioactive isotopes. [3,4] However, as thermoelectric technology has improved and the desire to decrease carbon emissions has increased, it has been proposed that thermoelectric materials could be used to recover energy from the waste heat produced by car engines. [4,5]
The Seebeck effect, in which temperature differences are converted into electricity, is caused by the diffusion of charge carriers of different temperatures. Hot carriers flow towards the cooler side of the material, and cold carriers towards the hotter side, which results in an electric current. This flow of both heat and charge will eventually equilibrate the temperature throughout an isolated device, but if the temperature difference is maintained the carriers will diffuse at a constant rate. If, then, the diffusion of carriers in one direction is higher than in the other, there will be more charges on one side of the material than the other and a voltage difference will develop. This directional difference in scattering occurs when the scattering mechanisms in the material are energy-dependent, resulting in the hot and cold carriers diffusing at different rates.
The performance of a thermoelectric material is measured by the dimensionless figure of merit
| Z T | = | α2σ
T λ |
where α is the Seebeck coefficient, which measures the voltage induced for a given temperature difference, σ is the electrical conductivity of the material, T is the temperature, and λ is the thermal conductivity of the material. [6] Current materials have a ZT of approximately one, corresponding to a seven to eight percent heat conversion efficiency; researcher's goal of a ZT of two to three would correspond to an efficiency of 15 to 20 percent. [7] We can see that good thermoelectric materials have a high electrical conductivity and a low thermal conductivity, which can be summed up as being a "phonon-glass/electron-crystal." The best candidate materials to exhibit this phenomena are semiconductors with narrow bandgaps and high-mobility charge carriers. A thermoelectric power-generation or solid-state cooling device can then be made from an n-type and a p-type semiconductor joined into a circuit by metal electrical contacts, as seen in Fig. 1. [2] The optimization of these material properties is also highly dependent on the temperature at which the final device is meant to operate.
Both bulk and nanoscale materials are being researched to make thermoelectric devices. While the most common bulk devices are made from doped bismuth telluride, researchers are also studying doped alloys of zirconium, hafnium, and lead tellurides; half-Heusler alloys of nickel, tin, zirconium, hafnium, and titanium; zinc antimonides; layered oxides; and intermetallic compounds containing rare-earth elements. [2] Superlattices, made of thin stacked layers of different materials, also show great promise in enhancing the properties of the bulk materials described above through careful engineering of quantum phenomena. [8] Similarly, one-dimensional nanostructured materials are also being researched for quantum size effects which enhance thermoelectric performance. These materials include carbon nanotubes, molybdenum, tungsten, and titanium sulfide nanotubes, and nanostructures of the bulk materials above. [9] However, more research is needed throughout the thermoelectrics field to develop materials capable of efficient energy generation from waste heat sources. [2]
© Sage Doshay. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] E. Velmre, Thomas Johann Seebeck (1770-1831)," Proc. Estonian Acad. Sci. Eng. 13, 276 (2007).
[2] T. M. Tritt and M. A. Subramanian, "Thermoelectric Materials, Phenomena, and Applications: A Bird's Eye View," Mat. Rev. Soc. Bull. 31, 188 (2006).
[3] H. V. Gaskill, "Method of Using the Peltier Effect for Cooling Equipment," U.S. Patent 2984077, 16 May 61.
[4] J. Yang and T. Caillat, "Thermoelectric Materials for Space and Automotive Power Generation," Mat. Res. Soc. Bull. 31, 224 (2006).
[5] J. M. Weisse, "Thermoelectric Generators," Physics 240, Stanford University, Fall 2010.
[6] D. M. Rowe, Thermoelectrics Handbook: Macro to Nano (CRC Press, 2005).
[7] T. M. Tritt, H. Böttner and L. Chen, "Thermoelectrics: Direct Solar Thermal Energy Conversion," Mat. Res. Soc. Bull. 33, 366 (2008).
[8] H. Böttner, G. Chen and R. Venkatasubramanian, "Aspects of Thin-Film Superlattice Thermoelectric Materials, Devices, and Applications," Mat. Res. Soc. Bull. 31, 211 (2006).
[9] A. M. Rao, X. Ji and T. M. Tritt, "Properties of Nanostructured One-Dimensional and Composite Thermoelectric Materials," Mat Res. Soc. Bull. 31, 218 (2006).