 |
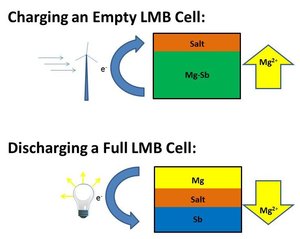
| Fig. 1: LMB charging and discharging mechanisms. |
Earlier this year on Comedy Central's faux news show, The Colbert Report, MIT professor Donald Sadoway, touted his invention of a liquid metal battery (LMB) that could drop oil prices to $20 a barrel, topple dictators, and bring world peace. [1] While those claims may seem farfetched, it should be noted that as an inventor, Sadoway stands to gain financially and therefore may be biased. So far, Ambri (Sadoway's battery start-up company), has not released the exact make-up of its LMB, but founders of the company have published research on a Magnesium-Antimony cell. [5] A battery of this type could potentially be used for grid-level energy storage that enables renewable energy sources like wind and solar to meet a much larger portion of world energy demand.
Wind and solar energy are both reliably unreliable. Compared to the exact calculations that can be made for traditional energy sources, renewables leave power companies scratching their heads. Knowing how much electricity a wind turbine or solar farm will produce is akin to predicting the weather. There is far less certainty and far more variability than managing, for example, the supply of natural gas to a gas turbine generator.
Beyond the inherent inconsistency of wind and solar sources of energy, there is the problem of matching power production with power demand. Traditional, predicable power sources can be ramped up or down according to demand. The amount of electricity produced from renewables is not coupled with demand. At night, when demand is low, wind generation is high. Extra energy from peak sunlight generation yesterday cannot be used tomorrow when it is cloudy. At least presently, there is no widespread method of storing renewable energy for future use, to match excess power production with future power demand.
If Sadoway's LMB is as amazing as we all hope, it would mean that large batteries could be feasibly employed in the power grid to store huge amounts of energy derived from wind and solar. Perhaps smaller batteries could be used at the local level to power individual homes with solar panel roofs and wind turbines in the backyard. Maybe LMBs could be used to power electric vehicles. That last scenario is definitely not likely, if we look at how the LMB was originally developed.
Ford Motor Company invented the Sodium-Sulfur (NaS) LMB in 1966, intending it for use in electric vehicles. [2] NaS batteries offer high power density and high energy capacity, but it should be noted the "LM" in LMB stands for "liquid metal," which means very high temperatures. NaS batteries typically are operated at around 290-390° C, which is a good reason for Ford to scrap the project. [3] A car crash involving very hot liquid metal spilling around sounds like an invitation for lawsuits. While it may be possible to reduce operating temperatures below 290° C, the liquid metal setup would seem to stand in the way of any LMB not using mercury, an element which would bring about more health and environmental concerns.
The more relevant application of LMBs then is to the problem of large storage devices where high operating temperatures pose less of a problem. NGK in Japan is a company doing just this. In 2002, an American electric company tested an NGK battery system for powering an office building in Ohio to test performance and economic viability. [3] Sandia Labs created a report based on this study and detailed in voluminous fashion that the battery worked, but the financial aspects are obscure and hard to follow. [3] The conclusion, in layman's terms, appears to be that if an NaS battery system were employed by an individual consumer for a large demand structure, like an office building, there could be potential cost savings by taking power from the grid at off-peak times when electricity is cheaper, storing it in the NaS battery, and then draining the NaS battery during peak-demand times when electricity is more expensive. The key word in that last sentence is "could." There are many caveats in the report based on region, season, how electricity pricing varies between peak and off- peak demand - in short, it depends.
A much clearer economic assessment of the same NGK battery was offered by ,USA Today in 2007: "The battery costs about $2,500 per kilowatt, about 10% more than a new coal-fired plant". [4] Without knowing the specifics of the LMB produced by Ambri, it is not possible to know exactly what the storage cost is, but one would presume with the passage of five years and Sadoway's optimism, it would be lower than the NGK battery figures.
While the economics involved may be murky, it is easy to make an educated guess as to how Ambri's battery works. Sadoway has spoken about it on TV after all. [1] A technical explanation is provided in a 2012 publication written by one of Sadoway's graduate students working on the Magnesium-Antimony (Mg-Sb) battery upon which Ambri based its battery. [5]
The Mg-Sb battery detailed in that paper consists of molten Mg and Sb separated by a molten salt (MgCl2-KCl-NaCl), all at 700° C. [5] The materials in the Mg-Sb battery were chosen because of their low cost (approximately $5-7/kg) compare to previous LMB experiments. [5] Three layers naturally form due to density differences. As shown in Figure 1, when the battery is charged fully, there is a maximum amount of Mg electrode. As electricity is discharged (lighting a light bulb in the figure), Mg cations seep down through the salt layer to reach the Sb layer and form an Mg-Sb alloy. Adding current (from a wind turbine for example), breaks apart the Mg-Sb alloy and reforms the Mg electrode. Thus, the battery returns to its charged state. In the laboratory, the Mg-Sb battery was 69% efficient (comparing the amount of electricity needed to charge the cell versus the current generated when fully discharging the battery). [5]
While the exact nature of the Ambri battery is not publicly available, it can be surmised that the operation mechanism would allow for a lifespan that would be acceptable to industry. The molten nature of the Mg-Sb battery allows Mg cation transport through the salt layer, but it also increases battery life because the electrodes are continually being reconstituted. While the Mg-Sb battery was only tested for a week, earlier research on NaS type batteries show that they typically have a life of 2,500 cycles. [5,6]. The fact that the electrodes are continually forming is an advantage of this battery structure compared to using solid-state electrodes that could be damaged over time. An Ambri battery might also last longer because it presumably does not use sodium, which being a corrosive element likely degrades the battery components over time.
The Mg-Sb cell that Ambri based its design on has a lower efficiency than the NGK NaS battery (69% versus 89%). [5,6] The Mg-Sb battery also has an operating temperature that is approximately twice that of NGK's model. [2,4] Ambri's website is not a source that meets the criterion for an acceptable reference for this report, so that leaves the battery specifications an unknown at present. In order to be cost- competitive with NGK, Ambri's battery must likely have an advantage in lower production cost, beyond using low cost materials. Sadoway holds a patent from 1991 for an improved method for producing aluminum in bulk at low cost. [7] While it could be just idle speculation, it seems possible that such a method might be employed to produce LMB components at costs that are low enough to make Ambri's battery economical.
Again, the exact make-up of Ambri's battery is unknown, so comparing the efficiency and operating temperature of the Mg-Sb prototype may well be meaningless. If it's not, it's currently hard to see how the new LMB in any way out-performs the NGK model tested 10 years ago. Until Ambri's battery design or an actual prototype is released, everyone will have to take Sadoway's word that this going to bring about $20 oil and world peace.
© John Belanger. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] L. Beslie, "Liquid Metal Battery: Can We Invent Our Way out of Climate Trouble?" Christian Science Monitor, 24 Oct 12.
[2] Z. Wen et al., "Research on Sodium Sulfur Battery for Energy Storage," Solid State Ionics 179, 1697 (2008).
[3] B. L. Norris, J. Newmiller and G. Peek, "NAS Battery Demonstration at American Electric Power," Sandia National Laboratory, SAND2006-6740, March 2007.
[4] P. Davidson, "New Battery Packs Powerful Punch," USA Today, 5 Jul 07.
[5] D. J. Bradwell et al., "Magnesium-Antimony Liquid Metal Battery for Stationary Energy Storage," J. Am. Chem. Soc. 134, 1895 (2012).
[6] B. Roberts, "Capturing Grid Power," IEEE Power and Energy Mag. 7, No. 4, 32 (2009).
[7] D. Sadoway, "Apparatus and Method for the Electrolytic Production of Metals," U.S. Patent 4999097, 12 Mar 91.