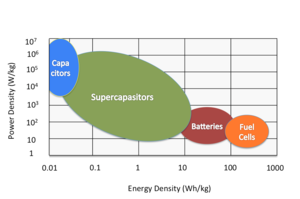
 |
| Fig. 1: Ragone plot of energy density vs. power density for various energy-storing devices. (after [3]) |
Electrochemical double layer capacitors, also known as supercapacitors or ultracapacitors, are energy storage elements with high energy density compared to conventional capacitors and high power density compared to batteries. Unlike conventional capacitors, where no chemical reactions is used and small amount of energy is stored by physically storing electric charges between two conductive plates upon application of an electric field, these electrochemical storage devices cross the boundary into battery technology by using special electrodes and electrolyte, and have capacitance values as high as 3500 Farads in a single standard case size with long cycle life (>100 000 cycles). [1]
Supercapacitors are the preferred choice in applications requiring a large amount of energy to be stored and delivered in bursts repeatedly. Batteries have high internal resistances (ESR) that are too large to continue to consistently deliver high-power pulses without increasing the risk of performance failures. Supercapacitors ultra-rapid charging and delivery of high current make them an ideal candidate to balance loads on power grids, standby/backup power supplies, or peak-load enhancer for hybrid vehicles. In these applications, supercapacitors will supply power to the system when there are surges or energy bursts, while the batteries can supply the bulk energy since they can store and deliver larger amount energy over a longer slower period of time. [2]
Very similar to batteries, supercapacitors energy storage mechanism is bulk separation and movement of charges. Supercapacitors are constructed from two electrodes, an electrolyte (aqueous or organic) and a separator that allows the transfer of ions, while providing insulation between the electrodes. Different materials such as various carbon materials, mixed metal oxides and conducting polymers have been used as supercapacitor electrode materials. [3] Carbon technology in its various forms such as carbon cloth, activated carbons and carbon nanotube are the most widely used electrodes. [4] In recent years graphene due to its superb characteristics of chemical stability, high electrical conductivity and large surface area has also been considered as a promising capacitor electrode material. [5]
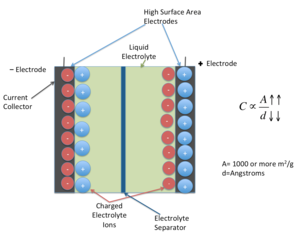 |
| Fig. 2: Schematic of a supercapacitor. (after [3]) |
As voltage is applied to a supercapacitor, ions in the electrolyte solution diffuse into the pores of the electrode of opposite charge. Charge accumulates at the interface between the electrodes and the electrolyte, forming two charged layers (double layer) with an extremely small separation distance. This is the distance between the electrode surfaces to the center of the ion layer. Carbon technology used in these capacitors creates a very large surface area with separation distance of just a few angstroms (0.1nm), due to their porosity. Since the capacitance value is proportional to the surface area and is the reciprocal of the distance between the two layers, high capacitance values can be achieved in a very small space. [1,6]
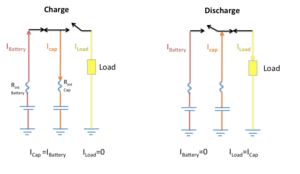 |
| Fig. 3: Battery powered pulse circuit. |
In addition to having a limited number of charge-discharge cycles, batteries performance especially at low temperatures are deteriorated and their life cycle is significantly shortened when a large pulsed discharging current with peak greatly exceeding the average power consumption happens. To reduce the peak current draw in battery powered electronics with the highly fluctuating load, supercapacitors are widely exploited to mitigate such load current fluctuations in the batteries. Devices that would require high-power pulses include GPS tracking systems, bluetooth communications devices, RFID equipment, medical equipment and alarm and security systems. Under pulsed load conditions, the supercapacitor acts as a filter that relieves peak stresses on the battery by supplying power to the system in short energy bursts. After delivering the pulse current, the supercapacitor is quickly recharged by the battery between the pulse cycles. Parallel battery-supercapacitor connection storage greatly enhances peak power, considerably reduces internal losses and extends the discharge life of the battery. [2,7]
Supercapacitors offer a promising alternative approach to meeting the increasing power demands of energy storage systems and electronic devices. With their high power density, ability to perform in extreme temperatures, and millions of charge-recharge cycle capabilities, supercapacitors can increase circuit performance and prolong the life of batteries. This can add value to the end-product and ultimately reduce the costs to the customer by reducing the amount of batteries needed and the frequency of the replacement of the batteries, which adds greatly to the environmental friendliness of the end-product as well.
© Marjan Aslani. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] J. R. Miller and A. F. Burke, "Electrochemical Capacitors: Challenges and Opportunities for Real-World Applications," Electrochemical Society Interface 17, No. 1 (Spring 2008), p 53.
[2] R. A. Dougal, S. Liu and R. E. White, "Power and Life Extension of Battery-Ultracapacitor Hybrids," IEEE Trans. Components and Packaging Technologies 25, 120 (2002).
[3] M. Winter and R. J. Brodd, "What Are Batteries, Fuel Cells, and Supercapacitors?" Chem. Rev. 104, 4245 (2004).
[4] L. L. Zhang and X. S. Zhao, "Carbon-Based Materials as Supercapacitor Electrodes," Chem. Soc. Rev. 38, 2520 (2009).
[5] Y. Wang et al., "Supercapacitor Devices Based on Graphene Materials," J. Phys. Chem. C 113, 13103 (2009)
[6] E. Frackowiak, "Carbon Materials for Supercapacitor Application," Phys. Chem. Chem. Phys. 9, 1774 (2007).
[7] D. Shin et al., "Battery-Supercapacitor Hybrid System for High-Rate Pulsed Load Applications," Design, Automation and Test in Europe Conference and Exhibition (DATE), 14 Mar 11.