 |
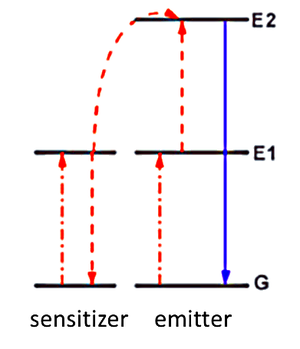
| Fig. 1: Energy levels and energy transfer processes involved in ETU. |
When a photon hits a semiconductor, an electron-hole pair is generated with the probability of η, i.e. the internal efficiency of the material. If collected properly, these carriers lead to an electric current. The process of converting solar power to electricity is called photovoltaics. Due to intrinsic loss mechanisms in semiconductors, solar cells suffer from fundamental efficiency limitations including: 1) Sub-bandgap energy photons with deficient energy cannot excite the electrons from the valence to the conduction band, 2) Above-bandgap energy photons lose their excess energy as heat, limiting the cell performance. While proper texturing of the cell surface, designing perfect absorbers, and anti-reflective coatings alleviate the reflection losses to enhance the cell efficiency, the issue of absorbing low energy photons is still a challenge. [1,2]
Up-conversion (UC), first recognized by Auzel, Ovsyankin, and Feofilov in the mid-1960s, is the nonlinear optical process of absorbing two or more low energy photons successively, exciting an electron to some intermediate state and ultimately raising it to a high-energy level. The excited electron then relaxes to the ground state by emitting a shorter wavelength photon. [3,4] Among various up-conversion processes the energy transfer up-conversion (ETU) is the most probable and fastest one widely used in practical applications. ETU is the process of populating E2 via sequential excitation from G to E1 and then to E2. The required energy to excite the electron from E1 to E2 is provided through a non-radiative relaxation process, transferring the energy between two adjacent ions in the system. In this case the exciting ion is called sensitizer and the other one, relaxing to the ground state via a radiation process, is called activator or emitter. Fig. 1 shows the energy levels and transfer process of ETU.
Started from lanthanum with the atomic number of 57, the lanthanide series also called the f-block, includes 15 elements in the periodic table. Their 4f orbitals participating in the optical transitions of rare-earth doped ions are well-shielded from the environment by full 5s and 5p orbitals. At the same time many of the electric and magnetic dipole transitions are parity forbidden between f orbitals leading to small absorption coefficients for such f-f transitions. These facts lead to long-lived excited states manifested by sharp lines in emission spectra of the lanthanide ions. With the exception of La3+, Ce3+, Yb3+, and Lu3+ the lanthanide ions commonly have more than one excited 4f energy level making them proper candidates for UC purposes. For example Er3+, Tm3+, and Ho3+ with ladder-like energy level characteristics have been frequently used as activators in ETU process. Also due to their energy level and spacing values these ions are able to convert the low energy, near infrared photons to visible ones making them a proper candidate for solar applications as well. Fig. 2 shows the energy levels and the transitions leading to UC emission in ER3+. In this ion the absorption occurs at 980 nm (IR) and the up-converted photons are emitted at 665 nm (Red), 521 nm and 542 nm (Green).
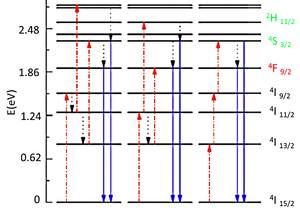 |
| Fig. 2: Energy level diagram of ER3+ and its UC processes. The dashed-dotted, dotted and full arrows correspond to excitation, multi-photon relaxation and emission processes, respectively. |
The optical properties of lanthanide-doped systems, their energy levels, emission profiles and their UC efficiency can be largely modified through nano-scale manipulation of nano-crystals. For example, the intermediate state lifetime can be modified via particle size reduction. The control over spatial confinement of dopant ions within a nano-scopic region can enhance the emission at a particular wavelength while generating new types of emission as well. [4]
Although in principle UC can be expected from most lanthanide-doped crystalline host materials, efficient UC only occurs in a small number of well selected and compatible dopant-host combinations. The host materials generally require close lattice matching to the dopant ions and low energy phonons. As all trivalent rare earth ions exhibit similar ionic size and chemical properties, their inorganic compounds are ideal host materials for up-converting lanthanide dopant ions. In addition, alkaline earth ions (Ca2+, Sr2+, and Ba2+) and some transition metal ions (Zr4+ and Ti4+) also exhibit close ionic size to lanthanide ones. [4] Therefore, inorganic compounds containing these ions are also frequently used as host materials for UC processes. However doping these nano-crystals with lanthanide is always accompanied by the formation of crystal defects such as interstitial anions and cation vacancies. As a single crystalline phase host is required for an efficient UC, the dopant concentration should be precisely controlled. Semiconductor nano-crystals such as ZnS are another candidate group to host UC materials. However due to the large mismatch between the size of the host and the dopant ions it still is unknown whether the lanthanide ions are mainly located on the outermost layer of the crystal or they are uniformly distributed in the host lattice. The literature survey shows that the most efficient UC systems are in fluoride hosts. So far NaYF4 seems to be the best host material with the efficiency of 3% in the bulk and 1% in the nano-particle form.
Having almost the 52% of sun energy flux being in the IR, the solar cell (SC) performance can be greatly improved if low energy photons are absorbed as well. A recent theoretical study on realistically modeled UCs demonstrates that the cell efficiency can be enhanced up to 44%. [5] In 2010 deWild reported the first application of NaYF4:Yb3+/Er3+ UC to the back of a Si-SC where they measured 10 μAcm-2 current density for 980 nm diode laser illumination. [6] While similar experiments prove that the lanthanide doped systems can efficiently up-convert 980 nm, low energy IR photons, to visible ones suitable for being absorbed by the active layer of SC it should be remembered that the external quantum efficiency of these UCs won't exceed 1%. Also as the energy level diagram in Fig. 2 implies the narrow bandwidth absorption and emission features of the lanthanide ions limit their efficiency for real AM1.5D solar spectrum. The low flux of the solar power in the absorption and emission range of Ln3+ doped UCs requires at least 10000X more efficiency for practical applications.
To harvest the sub-bandgap portion of the solar spectrum and enhance the efficiency and performance of the current solar cells, up-converter incorporated PVs have been proposed. Among different UC schemes, Ln3+ doped up-converters are one of the proper candidates as their energy levels match up with one of the solar spectrum. However their low quantum efficiency, less than 1%, and narrow bandwidth, are two major drawbacks of these systems making them challenging for practical applications. Nowadays various research trends on Ln3+ doped UCs are mainly focused on the external quantum yield enhancement of the rare-earth doped nano-particles as well as increasing the bandwidth. Aside from these challenges which are more related to UC part the incorporation of UCs in current SCs is another issue needed to be considered as well. After all optimizing the Ln3+-UCs for higher efficiencies and larger bandwidth combined with economic schemes for UC/ SC interfacing are the main problems needed to be resolved before utilizing lanthanide ions in commercial solar applications.
© Hadiseh Alaeian. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] S. Shu and Y. Y. Li, "Metallic Rugate Structures for Near-Perfect Absorbers in Visible and Near-Infrared Regions," Opt. Lett. 37, 3495 (2012).
[2] Y. Gong et al., "Perfect Absorber Supported By Optical Tamm States in Plasmonic Waveguide," Opt. Express 19, 18393 (2011).
[3] F. Wang and X. Liu, "Recent Advances in the Chemistry of Lanthaniede-Doped Upconversion Nanocrystals," Chem. Soc. Rev. 38, 976 (2009).
[4] F. Auzel, "Upconversion and Anti-Stokes Processes With f and d Ions in Solids," Chem. Rev. 104, 139 (2004).
[5] A. C. Atre and J. A. Dionne, "Realistic Upconverter-Enhanced Solar Cells With Non-Ideal Absorption and Recombination Efficiencies," J. Appl. Phys. 110, 034505 (2011).
[6] J. de. Wild et al., "Enhanced Near-Infrared Response of a-Si:H Solar Cells With β-NaYF4:Yb3+(18%),Er3+(2%) Upconversion Phosphors," Solar Energy Mat. Solar Cells 94, 2395 (2010).