 |
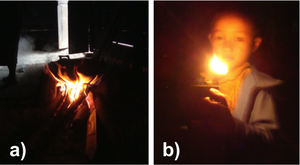
| Fig. 1: a) Cooking fire, Akha village; b) Boy with kerosene lamp, Hmong village, Lao PDR. |
Access to a reliable source of energy is one of the main factors affecting quality of human life. [1] While taken for granted in developed nations, the luxury of having dependable and abundant sources of energy is greatly desired in least developed and developing nations. In these energy-impoverished regions, common fuel sources include biomass (e.g. wood, charcoal) and fossil fuels (e.g. kerosene), which are burned for cooking and for use as a low-grade lighting source (Fig. 1). These fuels can be expensive, and chronic exposure to combustion byproducts has major economic and health consequences. [2] The demographic still utilizing energy in this way is at the Bottom Of the socioeconomic Pyramid (BOP) - approximately 2.5 billion people who live on less than $2.00 USD/day. [3]
The BOP has limited purchasing power and little chance to break the cycle of poverty. Appropriate technologies afford a means to improve standard of living, but the mechanisms to effect this change are complex. [4] Socioeconomic, cultural, technical, political, and environmental factors (to name a few) must be considered. Altering daily routines and habits often prove to be most challenging, even when economic gain can be realized. Successful technology integration, both large- and small-scale, relies heavily on intimate knowledge of and partnership with the target population.
This paper will draw on the author's experiences of working in rural, poor, non-electrified areas of Southeast Asia with Sunlabob Renewable Energy, Ltd. It will explore replacing kerosene usage for lighting with battery lanterns that employ a globally available energy resource, sunlight. The luminous efficacy of a select group of solar-powered battery lanterns will be compared with that of a kerosene flame in the context of appropriate technologies.
The unit "lux" is used to determine how well an area is illuminated at a certain distance from a source and is defined as the amount of visible light radiated per second in a solid angle (lumen or lm) that falls on a defined surface area. Basic fuel-based lighting, such as a simple kerosene wick lamp, only offer around 1 lux at a distance of 1 m, whereas 150 lux for studying and 300 lux for a living area are recommended. [5,6] The following will seek to elucidate why this widely used kerosene lamp produces such a low-light setting.
The combustion of long-chain hydrocarbons is complex. Many intermediate and final excited-state products form during this process, producing a seemingly continuous spectrum from the array of incandescent particulates (e.g. soot) and luminescent species. [7] This continuum can be modeled as blackbody radiation, which is dependent on temperature. For a kerosene flame, its absolute temperature cannot be used; instead, a Correlated Color Temperature (CCT) is calculated to mimic the emission spectrum of the light source. [8]
To get a sense of how efficient a kerosene flame is at producing visible light, its luminous efficiency - a ratio of visually stimulating radiation to all radiation emitted - is compared to that of the Sun. To quantify the amount of light at each wavelength, the spectral power distribution function JT(λ) - power radiated per unit area per wavelength from a surface - for these light sources was modeled by Planck's Law for blackbody radiation. JT(λ) was normalized so that integrated values fall between 0 and 1 and is
|
(1) |
where h is Planck's constant, c is the speed of light, kB is Boltzmann's constant, and T is temperature in Kelvin. The photopic luminosity curve V(λ) reflects the spectral sensitivity - luminous efficiency per wavelength - of the eye in bright light settings and has a maximum sensitivity (1.0 or 100%) at 555 nm. [8,9] Determining the luminous efficiency of the two light sources can be accomplished by integrating Eq. (1), weighted by V(λ), over all wavelengths
|
(2) |
Using the effective temperature of the Sun (5780°K) and the approximate CCT of an open kerosene flame (1800°K), the luminous efficiency was calculated for both sources by Eq. (2). [10,11] A kerosene flame is an exceptionally inefficient lighting source with around 0.59% of its radiation within the visible range of 380 - 750 nm as compared with nearly 44% from the Sun. [12] When considering the photopic sensitivity of the eye, their luminous efficiency values are 0.096% and nearly 15%, respectively.
Fig. 2 illustrates the shape of JT(λ) for each source normalized to λmax and their overlap with V(λ). Note that the Sun's non-normalized peak intensity is a little over two orders of magnitude larger than that of kerosene and that a Gaussian fit was used to approximate V(λ) for Eq. (2).
The luminous efficacy - a ratio of luminous power (perceived brightness) to all power radiated - of the two light sources can be estimated from Eq. (2) because by convention, V(λ) is defined so that the maximum sensitivity at 555 nm correlates with a value of 683 lm/W. [8] Thus, luminous efficacy values for a kerosene flame and for the Sun were estimated at 0.65 lm/W and 99 lm/W, respectively.
Battery-powered lanterns and flashlights offer an alternative to fuel-based lighting for remote, off-grid locations. These alternatives vary by source of energy, by energy storage medium, by emitting source, and by functionality. Here, we consider two solar-powered battery lanterns that have been offered by Sunlabob Renewable Energy in Vientiane, Lao PDR.
One lantern uses a Cold Compact Fluorescent Light (CCFL) bulb and a lead-acid battery, the other uses a Light Emitting Diode (LED) and NiMH batteries (Fig. 3). A comparison of energy storage density and luminous efficacy can be found in Table 1 along with a simple kerosene wick lamp.
|
||||||||||||||||||||||
| *Lower Heating Value (LHV) | **calculated from experimental values. [5,13] | |||||||||||||||||||||
| Table 1: A comparison of energy density and luminous efficacy of a basic kerosene wick lamp, a 4 W 120 lm CCFL lantern, and a 1.8 W 120 lm LED lantern. | ||||||||||||||||||||||
The theoretical luminous efficacy of a kerosene flame (0.65 lm/W) is eight times the value determined experimentally (0.08 lm/W). While there is uncertainty inherent in the measured and calculated values (especially the CCT), inefficient combustion by the kerosene wick lamp may be the main cause for this discrepancy. Comparing the luminous efficacy values to those of related lamps supports this supposition. A hurricane wick lamp uses a protective glass casing and controllable fuel release, whereas a simple kerosene wick lamp does not. A pressurized hurricane lamp vaporizes the fuel for combustion and an incandescent mantle uses the heat released to glow very brightly in the visible spectrum. The luminous efficacy values for both alternative fuel lamps are 0.11 lm/W and 0.80 lm/W, respectively - an improvement over the simple kerosene wick lamp. [5]
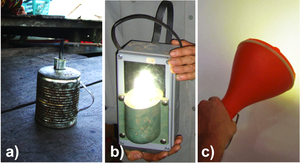 |
| Fig. 3: a) Kerosene lamp; b) Lao-made CCFL lantern; c) German-engineered LED lantern. |
Looking at Table 1, the relatively high luminous efficacy - a ratio of luminous power to power input, in this case - of the CCFL (30 lm/W) and LED (67 lm/W) light sources when compared with that of a kerosene wick lamp (0.08 lm/W) is inherent in their design. CCFLs make use of excited gas electron transitions that emit UV light. This light, in turn, excites phosphor-coated glass, which emits largely in the visible. White LEDs can utilize phosphors as well or can be composed of multilayered semiconductors, where each layers emits different wavelengths of light. The aggregate of colors produced can create white light.
Although kerosene stores three orders of magnitude more energy per kilogram than that stored in a solar lantern, the poor luminous efficacy of an open flame hinders its ability to perform against battery-powered alternatives. When a kerosene lamp is replaced with a CCFL light source, a marked difference in illumination intensity is obvious (Fig. 4a). In terms of functionality, cost, color quality, and weight, a further improvement can be obtained with an LED light source and high-energy density batteries (Fig. 4b).
It is important to note that the spectral sensitivity of the eye changes with lighting intensity. The eye likely operates in the mesopic regime for a kerosene wick lamp due to the low-light setting it creates (see Fig. 1b). [6,16] Mesopic vision is a combination of photopic (day) and scotopic (night) vision; the latter can be thought of as a blueshift of the wavelength of maximum light sensitivity by 50 nm. The resulting change in spectral sensitivity and thus, the luminosity curve would have a significant effect on luminous efficacy values.
 |
| Fig. 4: a) A comparison of the CCFL lantern with a villager's kerosene lamp at a floating village on Tonlesap Lake, Cambodia; b) an LED lantern in an Akha village, Lao PDR. |
All things considered, however, modeling a kerosene flame as a blackbody radiator provides a fairly accurate estimation of the amount of visible light given off relative to all emitted light.
One could argue that wood and fossils fuels are forms of solar energy that can be stacked or bottled, but directly producing visible light from the stored energy released in an open flame is very inefficient. Solar lanterns substitute photosynthesis with photovoltaics, where rechargeable batteries serve as the energy storage medium. By replacing kerosene lamps with more energy-efficient solar-powered battery lanterns, quality of life could be improved by decreasing health risks (e.g. fire, noxious fumes), providing higher quality lighting for studying at night, and increasing hours for income-generating activities. However, the difference in up-front costs between fuel-based and solar-powered lighting are largely responsible the slow market penetration of the latter. Further, changing behavioral usage and disrupting kerosene's well-established distribution network is no easy task.
Kerosene users pay a small initial investment for a kerosene lamp can and only need to pay manageable weekly payments for fuel. Reliable solar-powered battery lanterns have a much higher initial capital investment, which is a main deterrent for would-be buyers. This cost can be quoted at over $40 USD. In Fig. 5, the capital and operational costs of a $25 USD, 1 W LED lamp with focusing lens and NiMH batteries, a $1 USD kerosene wick lamp, a $3 USD hurricane wick lamp, and a $25 USD pressurized hurricane lamp with glowing mantle are compared using values from a 2005 study. [5]
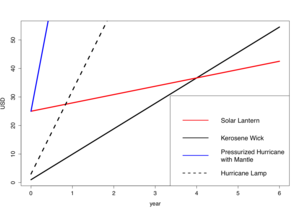 |
| Fig. 5: Accumulated costs of a solar lantern (red), a simple kerosene wick lamp (black), a hurricane wick lamp (dash), and a pressurized hurricane lamp with mantle (blue). |
The initial capital investment is higher for the LED solar lantern relative to the simple kerosene wick lamp. But, after approximately four years, the LED solar lantern reaches simple payback due to higher operating costs associated with the kerosene lamp. In the long-term, both hurricane lamps have prohibitively high annual operating costs. The solar lantern's return on investment period is somewhat long and also dependent on whether it can operate for over four years; nevertheless, the quality of life improvement needs to be factored in and often outweighs the cost issue. Thus, innovative financing mechanisms (e.g. locally tailored micro-loans) in concert with the development of low-cost, high-quality products must be considered.
Sunlabob creates public-private partnerships to provide the initial capital for its Solar Lantern Rental Systems (SLRS). These projects seek to establish a village-run microenterprise of renting solar-powered lanterns at prices comparable to or less than those of kerosene. All money stays within the village and these systems have had some success. Unfortunately, SLRS are funded by grants and by private donations that are not reliable, long-term funding streams. Luckily, however, there is money to be made in the developing world. [17] A well-capitalized, for-profit company with an affordable, quality product for the BOP could sell it to hundreds of millions. Low margins are overcome by the shear size of the market. Whether introducing a new product or integrating an idea into an existing technology, a fortune is waiting out there for innovative entrepreneurs. Rural lighting products are not likely to be an exception.
© Michael Machala. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] G. Legros et al., "The Energy Access Situation in Developing Countries, World Health Organization, November 2009.
[2] N. T. K. Oanh, L. H. Nghiem and Y. L. Phyu, "Emissions of Polycyclic Aromatic Hydrocarbons, Toxicity, and Mutagenicity from Domestic Cooking Using Sawdust Briquettes, Wood, and Kerosene," Environ. Sci. Technol. 36, 833 (2002).
[3] "Poverty Poverty Data: A Supplement to World Development Indicators 2008," The World Bank, December 2008.
[4] P. K. Ghosh, Appropriate Technology in Third World Development (Greenwood Press, 1984).
[5] E. Mills, "The Specter of Fuel-based Lighting," Science 301, 1263 (2005).
[6] S. Nandi and S. Sawkar, "Intensity of Artificial Lighting in Living Room and Study Area of Urban Residential Homes in Dharwad City," J. Hum. Ecol. 21, 63 (2007).
[7] M. W. Thring, The Science of Flames and Furnaces (Wiley, 1952).
[8] S. H. Schwartz, Visual Perception: A Clinical Orientation (McGraw-Hill, 2004).
[9] "Colorimetry - Part 1: CIE Standard Colorimetric Observers," ISO 11664-1:2007, Intl. Organization for Standardization, 2007.
[10] B. Jones, Life in the Solar System and Beyond (Praxis Publishing, 2004), p 7.
[11] Commission Internationale de l'Éclairage Proceedings (Cambridge, 1932), p. 301.
[12] C. Starr, C. A. Evers and L. Starr, Biology Concepts and Applications, 8th Ed. (Brooks Cole, 2010).
[13] N. Iqbal and M. H. Salley, "Fire Dynamics Tools (FDTs): Quantitative Fire Hazard Analysis Methods for the U.S. Nuclear Regulatory Commission Fire Protection Inspection Program," U. S. Nuclear Regulatory Commission, NUREG-1805, December 2004, pp. 3-4, 3-13.
[14] D. Pavlov, Lead-Acid Batteries: Science and Technology (Elsevier, 2011), p. 2-107.
[15] T.-K. Ying et al., "Studies on Rechargeable NiMH Batteries," Int. J. Hydrogen Energ. 31, 525 (2006).
[16] A. Stockman and L. T. Sharpe, "Into the Twilight Zone: the Complexities of Mesopic Vision and Luminous Efficiency," Ophthal. Physiol. Opt. 26, 225 (2006).
[17] C. K. Prahalad, The Fortune at the Bottom of the Pyramid (Pearson Education, 2006).