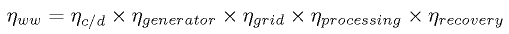
|
||||||||
| Table 1: Battery efficiencies [1-3] |
Electric cars seem to be a new trend recently, with many car companies rolling out their own version of an all-electric car. Interestingly, electric cars have been around for many years; however, most of these cars had their own limitations and did not eventually succeed in the marketplace.
Recently, more and more companies have been pushing towards using lithium-ion batteries in their electric cars. The Tesla Roadster is an all-electric car that has been gaining some popularity, due to its sleek sports car design, and its touted battery efficiency. Nissan is coming out with a new car, the Nissan Leaf, which is very similar to the Roadster with a Li-Ion battery pack, but with a short range of 100 miles per full charge. The Chevy Volt is another contender to the stage, which is actually a hybrid which uses a gasoline engine generating electricity to the electric motor.
It was actually very hard to find much information on the internet, about how efficient these car batteries really are. Adding to this lack of information was the fact that there are many different ways to calculate efficiency, and that there are many factors that can be overlooked. The efficiency of a battery can be calculated as the amount of power discharged by the battery divided by the amount of power delivered to the battery. This takes into account the loss of energy to heat, which warms up the battery. The charge-discharge efficiencies of various batteries are summarized in Table 1. Li-ion efficiencies are extremely high, Pb-acid efficiencies have a huge range, NiMH efficiencies are low at 66%. [1-3]
Unfortunately, the charge/discharge efficiency of a battery tells us next to nothing about the real efficiency, because we have to take into consideration where the energy is coming from, and how efficient it is to convert our starting fuel into electricity. A good way to look at the real efficiency is to consider the energy from the beginning natural gas generator, all the way to charging the car's battery. The cumulative losses are given roughly by the "well-to-wheel" efficiencies
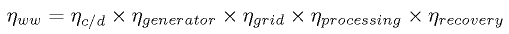 |
(1) |
listed in Table 2. Note that the the most efficient natural gas generator, the GE H series gas turbine, is 60% efficient at converting gas into energy. Thus, for example, from the Tesla Roadster's official energy consumption of 2.53 km/MJ we obtain an "effective" energy consumption of
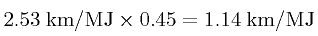 |
(2) |
As I took these figures from an article written by the founders of Tesla, it comes to no surprise that the Roadster shines as the most energy efficient car to date.
|
||||||||||||||||||||||||||||
| Table 2: Well-to-wheel battery efficiencies as given by Eqn. (1). The charge-discharge efficiency ηc/d is from Table 1. |
However, there are many confounding variables other than the charge/discharge efficiency that must be considered when comparing different battery types. For instance, the Li-Ion batteries have a much higher energy density than Pb-Acid batteries, and slightly more than NiMH batteries. This means that for the same weight of batteries, the Li-Ion batteries will be able to produce much more energy, which is a big factor in making car batteries. One of the reasons the Tesla Roadster is so efficient is because its battery is relatively light, which leads to a better gas mileage in general. Using a super efficient lead-acid battery would technically be more efficient, but its weight would severely decrease the gas mileage of the vehicle.
There is another factor that we have to consider, which is that these batteries all depend on a source of energy to be charged. For the calculations above, we have been assuming that we are using the most efficient way to get electricity, with a natural gas turbine. With an average generator, we would most likely see a significant drop in efficiency. Regardless, electric cars do seem to outstrip gasoline cars in terms of going the most miles for the least amount of energy. Using the Honda Civic as an example, we can calculate what the well-to-wheel efficiency is. For gasoline, transferring the fuel from the source to the car is actually more efficient than converting natural gas into electricity. From the source to the car, gasoline actually has an efficiency of 81.7%. [4] This seems to blow the electric car out of the water, but the usage of the gasoline in the car decreases the efficiency drastically, because gasoline cars have a very low mileage. The Honda Civic is a car with one of the highest gas mileages, but its 51 mpg is low compared to the energy usage of the Roadster.
In conclusion, electric batteries are more efficient at using energy than regular gasoline engines, but other factors such as convenience and price prevent the electric cars from becoming widely used. If these hurdles can be overcome, the electric car has the potential to compete, and potentially even replace gasoline cars altogether.
© John Sun. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] M. F. Cowlishaw, "The Characteristics and Use of Lead-Acid Cap Lamps," Trans. British Cave Research Association 1 199 (1974).
[2] Y. Idota et al., "Tin-Based Amorphous Oxide: A High-Capacity Lithium-Ion-Storage Material," Science 276, 1395 (1997).
[3] M. A. Fetcenko et al., "Recent Advances in NiMH Battery Technology." J. Power Sources 165, 544 (2007).
[4] D. Sperling and K. Kurani, eds., Transportation, Energy, and Environmental Policy: Managing Transitions, Transporation Research Board of the National Academies, September 2001.