With innovations such as electric vehicles as well as laptop computers and mobile phones, we have an increasing dependence on rechargeable batteries. Many of these technologies, especially the electric vehicles, boast high efficiency and environmental friendliness, since energy conversion in batteries does not have the limits in traditional heat engines, and the vehicles do not emit gaseous pollutant. However, can the battery based technologies really lower our ecological foot print while maintaining material comfort? Let's take a step back to examine the batteries and their environmental impact before fully embracing those innovations. Let's discover the general principles behind a working rechargeable battery, specifics of electric car batteries as well as their environmental impact.
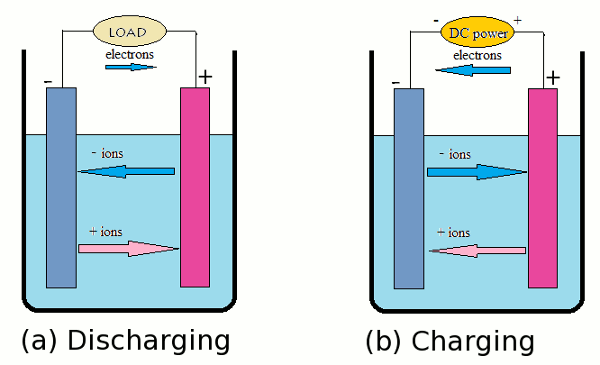
The rudimentary principles of a cells, working unit within batteries, have changed little over the centuries. A cell needs negative electrode, positive electrode, and electrolyte to be functional. A good negative electrode is a stable conductor that can be easily manufactured at a low cost, while a positive electrode needs to be stable in contact with the electrolyte and an effective oxidizing agent. An electrolyte, on the other hand, is an insulator that has high ionic conductivity. It also has to be cheap, safe to handle, and inert to temperature fluctuations. Most importantly, an electrolyte cannot react with the electrodes. The electrolyte is usually isolated from the rest of the battery, so the electric circuit is incomplete so the cell can retain its energy without discharging. Once a load is connected, electrons will flow from the oxidized negative to the oxidizing positive electrode, and reduce its material. The electrolyte then becomes activated as positive ions can now flow to positive electrode while the negative ions flow in the other direction (Fig 1.a). This process is reversed during charging (Fig 1.b), where an external power source encourages the ions to flow in a direction opposite to that of discharging, and the positive electrode is then oxidized. [1] In the rest of the paper, we will look at these components in different types of cell, and study their environmental impact.
 |
| Fig. 1: Schematic diagraom of working rechargeable batteries. |
Nickel-metal-hydrate (NiMH) batteries are commonly used in hybrid electric vehicles such as Toyota Prius, but there are also small NiMH batteries on the market. These batteries' positive electrodes are made of nickel oxyhydroxide, while their negative electrodes utilize hydrogen stored as metal-hydrate. The electrolyte in this type of batteries are usually potassium hydroxide solutions, and the nickel oxyhydroxide reacts to form nickel hydroxide during discharge [2]. We have several toxic materials in this reaction: potassium hydroxide is an acutely poisonous substance whose ingestion can cause "severe pain, vomiting, diarrhea and collapse, while nickel hydroxide is confirmed to cause cancer in humans and animals. [3] If the chemical is left in the environment to decompose, it will release toxic gases and vapors such as nickel carbonyl. [4] Another source of concern in NiMH batteries comes from the metal alloy it uses to hold hydrogen: the most common alloy is out of rare earth material such as lanthanum nickel whose toxicity has not been thoroughly investigated, although pure lanthanum and nickel are moderately to highly toxic. This lends us sufficient reasons to remain cautious about their compound. Given the toxic material it contains, the NiMH batteries may not be perfectly benign to the environment.
Another type of popular batteries are out of lithium-ion. They are used in devices such as iPhone 4G, plug-in hybrid vehicles, and full electric vehicles by Tesla Motor. These batteries' positive electrodes are from materials that can produce lithium-ion, while the negative electrodes are from elemental lithium intercalated on graphite [5]. Lithium ions are "widely distributed in nature; trace amounts are found in many minerals, in most rocks and soils, and in many natural waters," and FDA approves the use of ionic lithium in drugs [6,7]. Similarly, neither elemental lithium nor graphite are toxic, and their combination eliminates the volatile nature of metallic lithium. The electrolyte can be made out of many materials, but LiPF6 in carbonate solvent is the most common. Lithium carbonate can be a toxin to humans and animals, while LiPF6 can react with water to produce hydrofluoric acid, which is a major pollutant and contact poison. However, considerable amount of R&D has gone into improving the electrolytes: both polymer and ceramic materials are hopeful candidate for the next generation of lithium-ion batteries. As of the moment, lithium-ion cells have the least environmental impact in the battery family.
From our quick review, the electric vehicles' batteries do not seem to be as zero impact as we would like. Unlike the lead-acid batteries in traditional motor, both NiMH and lithium-ion batteries are classified as non-hazardous except in California, where all batteries need special treatment. Because of this policy, approximately 1.15 x 105 tonnes of nickel is released to the environment as of 2008. Although this is only about one third of the 3.4 x 105 tonnes of lead lost in the environment in the same year, it may still contribute to an environmental crisis if we convert all of the vehicles in the world to electric power. [8] Therefore, it is better to remain cautious about large scale implementation of electric vehicles until we have mastered reliable ways to recycle their batteries.
© Rebecca Nie. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] D. Linden, "Basic Concepts," in Handbook of Batteries 3rd Edition, ed. by D. Linden and T. B. Reddy (McGraw-Hill 2002).
[2] D. Linden and Doug Magnusen, "Portable Sealed Nickel-Metal Hydride Batteries," in Handbook of Batteries 3rd Edition (McGraw-Hill 2002).
[3] "Potassium Hydroxide," Hazardous Substances Data Bank, U.S. National Library of Medicine, CASRN 130-58-3.
[4] "Nickel Carbonyl," Hazardous Substances Data Bank, U.S. National Library of Medicine, CASRN 13463-39-3.
[5] G. M. Ehrlich, "Lithium-Ion Batteries," in Handbook of Batteries, 3rd Edition, ed. by D. Linden and T. B. Reddy (McGraw-Hill 2002).
[6] C. W. Kamienski, D. P. McDonald and M. W. Stark, "Lithium and Lithium compounds," in Kirk-Othmer Encyclopedia of Chemical Technolology, 4th ed. 15 (Wiley, 1995), pp. 434-463.
[7] "Ionic Lithium," Hazardous Substances Data Bank, U.S. National Library of Medicine.
[8] J. F. Papp, "Recycling - Metals," in 2008 Minerals Yearbook, U.S. Geological Survey, Department of the Interior, September 2010.