 |
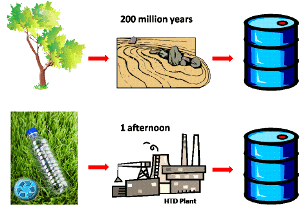
| Fig. 1: Comparing natural TD and HTD. (Clip art per site licence.) |
As the known oil reserves continue to dwindle, the need for alternate and/or renewable fuel sources is growing. Hydrous thermal depolymerization (HTD) is an aqueous temperature and pressure regulated process that's used to break down long hydrocarbon polymers found in organic matter into various products, including crude oil.
Thermal depolymerization (TD) occurs in nature when accumulated biomass is heated and pressurized in the earth's crust over millions of years. The biomass, also known as kerogen, is hypothesized to react with clay mineral enzymes [1] at temperatures < 200° C, a reaction which produces the oil people use today.
In the late 1970s, researchers began investigating the concept of hydrous pyrolysis, where super heated water catalyzes depolymerization. One of the first such experiments involved heating a volume-to-volume mixture of water and kerogen-rich shale to 330° C for a few days, after which a layer of oil would form on the surface. The oil produced under these conditions is similar in chemical makeup to that of naturally occurring oil, even though it is achieved using disparate means. [2] At sub-critical temperatures, water is known to exhibit dissolving properties similar to room temperature acetone, offering further support for its ability to break down kerogen. [3] Early on, this method was used as a means for investigating the science underlying natural oil formation. Currently, entrepreneurs are optimizing the HTD process as a means for producing clean oil, fertilizer, and other useful chemicals.
While the optimization of large-scale HTD processes is far from being achieved, one of the first such attempts, published in 2004 by Renewable Environmental Solutions, LLC (RES), is outlined below: [4]
Feedstock (biomass to be processed, typically organic waste) is fed into a chamber where it is mixed with water and heated to temperatures between 200° and 300° C. Contents are kept under high pressure.
The water-feedstock mixture is then subjected to a rapid reduction in pressure, allowing the first stage oil and water to separate, as well as allowing the removal of volatile gases which are harnessed and used for heating a boiler or spinning a turbine.
The final stage involves heating the remaining oil to temperatures near 500° C, allowing the first stage oil to separate into light hydrocarbons.
(See US patent 5,269,947 for a detailed illustration of a HTD apparatus.) RES claimed that feedstock could consist of any form of waste (plastic bottles, old electronics, sewage sludge, turkey offal, etc.). RES also claimed that there energy efficiency , defined as the total energy in the combustible products divided by the total energy input (energy in feedstock, electrical/natural gas expenditures) was close to 85%. [4]
According to National Recovery Technologies Inc., post-consumer plastics are estimated to represent 12% (30 Megatons) of the total municipal solid waste mass in the United States [5]. The energy density of a typical plastic is ~40 MegaJoules/kg [6]. In general, one expects impurities to reduce this value (here we assume it is reduced by a factor of 2). Hence, the total energy content in US plastic waste is currently
Globally, it is estimated that < 10% of plastic waste is recycled. [7] As an alternative to standard recycling, HTD could provide a means to convert this plastic to crude oil, with high efficiency.
© Derek Mendez. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] W. D. Johns, "Clay Mineral Catalysis and Petroleum Generation," Annu. Rev. Earth Sci. 7, 183 (1979).
[2] M. D. Lewan, J. C. Winters and J. H. McDonald, "Generation of Oil-Like Pyrolyzates from Organic-Rich Shales," Science 203, 897 (1979).
[3] M. Siskin abd A. R. Katritzky, "Reactivity of Organic Compounds in Superheated Water: Geochemical and Technological Implications," Science 254, 231 (1991).
[4] T. N. Adams et al., "Converting Turkey Offal into Bio-Derived Hydrocarbon Oil with the CWT Thermal Process," Changing World Technologies, Inc., 1 Mar 04.
[5] "High-Speed Plastic Recycling," National Recovery Technologies Inc.
[6] P. A. Kittle, " Alternate Daily Cover Materials and Subtitle D - The Selection Technique," Rusmar Inc., June 1993.
[7] M. P. Stevens, Polymer Chemistry: an Introduction" (Oxford U. Press,1999).