The dramatic climate change taking place around the world has been largely attributed to the unprecedented rise of the CO2 concentration in the atmosphere. Much effort has been made to develop alternatives to fossil fuels. However, fossil fuel will probably continue to dominate in the next few decades. Accordingly, there is good reason to consider retrofitting the current fossil-fuel-burning stationary power system to lessen the damage it causes. In the near term, despite of the massive implementation of clean energy, there might still be a large number of CO2-emitting facilities in the world, such as chemical plants. There is also a need to address the already high level of CO2 concentration in the atmosphere. Carbon Capture and Sequestration (CCS) is a promising method accomplishing all of these things.
The basic idea of CCS is to capture CO2 and other acid gases from the waste gas emitted by stationary facilities, compress them into supercritical state and inject them into a subsurface reservoir, such as depleted hydrocarbon reservoir, coal bed or brine aquifer. The technologies implementing these processes are quite mature. However, there is much debate and research on the long-term consequences ofe CO2 trapped underground. Also, the high cost associated with CCS has caused policymakers to rethink it vis-a-vis other clean energy techniques. Let us nonetheless briefly discuss some technical aspects of CCS.
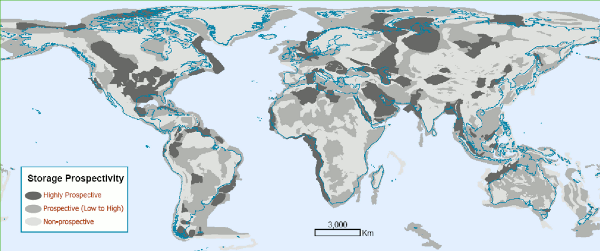
As is the case with hydrocarbon reservoirs, sedimentary rocks make the most suitable kind of CO2 storage, on account of their high porosity and permeability. Fig. 1 shows the distribution of sedimentary rock around the world. [1] These sedimentary basins can be oil and gas reservoirs (depleted or not), deep saline aquifers or coal beds. CCS may thus also help to recover natural gas, petroleum or coal bed methane.
In 2007, the world-wide annual energy-related CO2 emissions were about 30 Gtons. The figure for the USA was 6 Gtons. According to the U.S. National Energy Technology laboratory, the USA has CO2 storage capacity of 142.9 Gtons in oil and gas reservoirs, 188.0-217.5 Gtons in unminable coal formations and 3619.5-13459.0 Gtons in saline aquifers. Therefore, in total, these geological formations can potentially store over 1000 years of current CO2 emissions from power generation, far more than the actual needs. Due to the abundance in availability, CO2 storage in saline aquifers is the most studied storage form.
 |
| Fig. 1: Map of global geological carbon storage prospectivity. (Courtesy of the IPCC. Reported in IPCC 2005 after [1].) |
Currently, CO2 capture from emissions from power plants is done by absorption-desorption cycling with amine in form of solution or solid. Since this process separates CO2 with other components in the emissions and reduces the entropy, a certain amount of energy is required. The minimum energy required by the the second law of thermodynamics is [3]
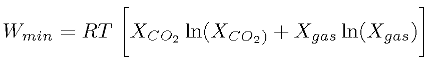
where XCO2 is the mole fraction of carbon dioxide and Xgas is the mole fraction of other gases without carbon dioxide. The higher concentration of CO2 is in the emissions, the less energy is required. This figure is around 200 kJ/kg CO2 captured for natural gas burning power plants but 500 kJ/kg for CO2 separated from ambient air. Considering the prohibitively high energy consumption for separating CO2 from air, this practice is only feasible when the CO2 concentration in air is intolerably high. For power plants, the energy required to separate CO2 from emissions can be directly derived from the boilers in the plants. However, the retrofitting of the infrastructure in power plants requires intensive capital investment, which comprises most of the cost of CCS.
CO2 can be transported by pipelines or ships. For maximal efficiency, the density of CO2 should be that of a liquid during transport. [4] However, the conditions are different for both cases. Because the pipelines are very long, the transport temperature of CO2 has to be close to ambient soil temperature, ranging from a few degrees below zero to 20 °C. [5] If the pressure of CO2 is maintained over the critical pressure of 7.38 MPa, there will be no gaseous CO2 in the pipeline from temperature fluctuations. For shipping, the facilities on the vessel can keep the CO2 quite cold and reduce the pressure of CO2. The temperature in that case is generally around -54 to -50 °C and the pressure is around 0.6 to 0.7 MPa. [6]
After transportation to the injection site, CO2 is pressurized and injected into designated depth through a well. This technique is comparable to those used in Enhanced Oil Recovery (EOR). The injection pressure and depth are critical for the storage of CO2. One should notice that the underground pressure and temperature both rise with depth. The geothermal conditions vary from place to place, and the operator must enture that CO2 will remain in the supercritical condition in the reservoir. This means that the CO2 should be injected at a depth of 800 m or greater.
Because CO2 is suffocating and makes water acidic when it dissolves, its leakage to the ground surface and interaction with underground water could have severe environmental consequences. The injection sites are thus chosen in which the geological formations provide several seals, consisting of shale with very low permeability, over the reservoir. [7] However, in the presence of faults, CO2 may move upwards through faults into shallow aquifers that supply drinking water or even to the ground surface. Water with dissolved CO2 can be moderately acidized and may dissolve hazardous constituents such as As, Pb from minerals. It may also displace saline water and hydrocarbons from deep reservoirs to degrade the shallow aquifer. If CO2 leaks to the ground surface, high concentrations of CO2 may harm the local ecosystem by suffocating plants and animals nearby. It would also put CO2 back into the atmosphere. The high pressure of CO2 can also damage structures if present. The risks are even higher if CO2 is co-sequestered with other hazardous gas such as H2S and SO2, since they are more poisonous and caustic in solution.
In order to reduce the potential risk of CO2 sequestration, investigators have proposed to trap CO2 in reservoir pore space or dissolved it in saline aquifers "immediately" after injection. Nowadays, risk assessment and trapping mechanisms are among the most popular topics in the area of CCS.
CCS is a potential remediation to stationary CO2 point source. It has huge capacity yet very high cost right now. [6] It is based on mature techniques but the risk assessment is still under investigation. Like other clean energy projects, the implementation of CCS will depend on government policies.
© 2010 Wenshi Chen. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] J. Bradshaw and T. Dance, "Mapping Geological Storage Prospectivity for the World's Sedimentary Basins and Regional Source to Sink Matching," in Proc. 7th Intl Conf on Greenhouse Gas Control Technologies, ed. by M. Wilson et al. (Elsevier, 2005).
[2] "Carbon Sequestration Atlas of the United States and Canada, 3rd Edition (Atlas III)," U.S. National Energy Technology Laboratory, 2010.
[3] G. A. Meehl et al., "Global Climate Projections," in Climate Change 2007: The Physical Science Basis, Intergovernmental Panel on Climate Change (IPCC, 2007).
[4] Z.-X. Zhang et al., "Optimization of Pipeline Transport for CO2 Sequestration," Energy Conversion Management 47, 702 (2006).
[5] S. McCoy and E. S. Rubin, "An Engineering-Economic Model of Pipeline Transport of CO2 With Application to Carbon Capture and Storage," Intl. J. Greenhouse Gas Control 2, 219 (2008).
[6] B. Metz et al., eds, IPCC Special Report on Carbon Dioxide Capture and Storage (Cambridge U. Press, 2005).
[7] S. Bachu, "Sequestration of CO2 in Geological Media: Criteria and Approach for Site Selection in Response to Climate Change," Energy Conversion and Management 41, 953 (2000).