 |
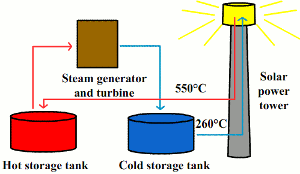
| Fig. 1: Schematic diagram of Solar II. |
One of the primary criticisms of renewable energy is its intermittent production. Whereas fossil fuels can be burned in direct response to electricity demand, most renewable energy production relies on favorable weather conditions. For this reason, hydropower is much more prolific than other renewable energy sources such as solar and wind since dams can be constructed in places where water flows year round.
If there is ever to be a world powered chiefly by renewable energy, efficient methods of storing energy will need to be developed and implemented. In this sense, hydroelectric power again has the advantage amongst the renewables since water can be pumped uphill and released back down during times of high demand. However, since the majority of prime hydroelectric sites in the world have already been used, other renewable energy sources will have to be utilized. [1]
Although many different energy storage devices, such as systems using batteries, flywheels, or compressed air, to be used in conjunction with solar photovoltaics and wind energy have been proposed, none of these systems can store large amounts of energy at reasonable costs or efficiencies. [2] When harnessing solar thermal energy, however, energy can be stored effectively in molten salts and at costs that are becoming increasingly competitive. [3] This feature of solar thermal power plants could enable them to provide steady baseload power that covers a significant portion of the energy demand.
Thermal energy from the sun can be stored either as latent heat or sensible heat. Sensible heat has to do with the heat capacity of a material. The added thermal energy stored in a material manifests as an increase in temperature. Latent heat is heat that is transferred due to changes in the phase of a material. [4] During a phase change, the material's temperature does not increase; energy is transferred in order to break or form intermolecular forces. Phase changes could be solid to gas transformations or liquid to gas transformations although the large change in volume associated with these changes make energy storage devices utilizing them difficult to manage. Solid to liquid transformations (melting) or solid to sold transformations, in which the crystal structure of a materials rearrange, are much more practical. [5]
Latent heat systems usually have high energy storage densities when compared to sensible heat storage devices. This is because the enthalpy change associated with phase changes is large compared to the sensible heat stored in a material across a typical temperature range. The enthalpy released when acetone freezes, for example, is 98 kJ/kg. [6] The specific heat of acetone, however, is 2.2 kJ/kg-°C, which means that the temperature of acetone must decrease by about 44 degrees to release an equivalent amount of enthalpy. [6]
The high energy densities of latent heat storage systems make them useful, but they must be applied to systems in which it is acceptable for the temperature of the heat source to be constant and for the heat storage material to solidify. Many heating pads take advantage of the heat of crystallization associated with sodium acetate transforming to sodium acetate trihydrate. [7] Houses with thermal storage units have been constructed using sodium sulfate, or Glauber's salt. Sodium sulfate decahydrate releases its water of crystallization to form anhydrous sodium sulfate at 32°C, an ideal temperature for low grade solar heating applications. [8] A small house (740 sq. ft.) in Boston was constructed more than 60 years ago that could be heated for up to ten consecutive sunless days. It utilized 21 tons of Glauber's salt that was stored in closets and in the partitions between walls. [9]
The main disadvantages of phase changing materials are that they are expensive, their ability to store heat often diminishes after numerous cycles of use due to incongruent melting, and since they solidify, an additional heat transporting medium with a heat exchanger must be used. [5] These problems have largely confined latent heat storage devices to small applications or experimental works. Sensible heat storage systems utilizing molten salt mixtures, however, have successfully been implemented on a large scale for use in solar thermal power plants.
Solar Two, a now decommissioned solar thermal power plant located near Barstow, CA in the Mojave Desert, was the first plant to feature a molten salt storage system. [10] It was a 10 MW central power tower system that used a mixture of 60% sodium nitrate and 40% potassium nitrate. This mixture melts at about 220°C and does not decompose until it reaches temperatures greater than 600°C. [11] The power tower setup allows for high enough temperatures to be reached that the molten salt mixture is used directly as a working fluid (Fig. 1). During the day, cold salt at around 260°C is pumped from a storage tank to the power tower. Hot salt at about 550°C is generated in the tower and is used to produce steam in a steam turbine to generate electricity. Extra hot salt is pumped to a second storage tank. At night, this hot salt is pumped to the steam turbine to generate additional electricity. [12]
The Andasol power station is a 50 MW solar thermal plant in Southern Spain that began operating last year. Like Solar Two, it uses a two tank molten salt storage system with 60% sodium nitrate and 40% potassium nitrate. [13] However, instead of a power tower, Andasol uses parabolic troughs to focus sunlight on an organic working fluid. This fluid is a mixture of diphenyl ether and biphenyl that melts at 12°C and has a maximum operating temperature of about 390°C. [11] The efficiency of the Rankine steam cycle is limited by the upper working temperature of the oil. [11] For energy storage, the working fluid heats up the molten salt through a heat exchanger. A fully heated tank of molten salts allows for the power plant to operate at full capacity for 7.5 hours after the sun has set. Only 3-7% less electricity is generated when thermal energy is stored in the molten salt tanks and used to produce electricity later as compared to when the energy is used to generate electricity directly. In other words, the molten salt storage system has an efficiency of 93-97%. [13, 14]
The Solar Two and Andasol solar thermal projects have demonstrated that molten salts can provide effective large-scale thermal energy storage and turn solar thermal plants into a baseload electricity source. Several additional solar thermal plants equipped with salt storage are being built or planned in Spain. However, from a cost perspective, it is disadvantageous to operate a smaller solar thermal plant with storage over a larger one without storage. Molten salt storage has only been popular in Spain because the country has placed a 50 MW limit on a power plant's eligibility for an attractive feed-in tariff. [3] Thus, through the use of molten salt storage, the owners of solar thermal plants can sell additional electricity at night while still benefiting from favorable Spanish renewable energy policies.
Since solar thermal energy currently represents such a small fraction of electricity generation, utilities do not care when the electricity is being generated. [3] However, if solar thermal power plants began to represent a significant portion of electricity generation, then the value of baseload solar thermal energy will likely increase and molten salt storage systems may become essential.
© Christopher Barile. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] J. P. Randolph, et al., Energy for Sustainability: Technology, Planning, Policy (Island Press, 2008).
[2] R. Baxter, Energy Storage: A Nontechnical Guide (PennWell, 2006).
[3] E. Clarke, "Thermal Storage in the US: Soon to Be a Given," CSP Today, 18 Dec 09.
[4] L. Pauling, General Chemistry (Dover, 1970).
[5] H. P. Garg, et al. Solar Thermal Energy Storage (D. Riedel Publishing Company, 1985).
[6] D. R. Lide, CRC Handbook of Chemistry and Physics, 87th edition (Taylor and Francis Group, 2007).
[7] J. A. Pergler, "Crystallization of Supersaturated Sodium Acetate and the Temperature Dependence of the Autoionization Constant of Water," J. Chem. Educ. 72, 1027 (1995).
[8] D. E. Garrett, Sodium Sulfate: Handbook of Deposits, Processing, Properties, and Use (Academic Press, 2001).
[9] H. E. Howe, "Sun Furnace in Your Attic," Popular Science, 154, 107 (1949).
[10] D. Mills, "Advances in Solar Thermal Electricity Technology," Solar Energy, 76, 19 (2004).
[11] R. W. Bradshaw, et al., "Molten Nitrate Salt Development for Thermal Energy Storage in Parabolic Trough Solar Power Systems," Sandia National Laboratories, ES2008-54174 (2008).
[12] M. Valenti, "Storing Solar Energy in Salt," Mechanical Engineering, 117, 72 (1995).
[13] B. Moselle, et al. Harnessing Renewable Energy in Electric Power Systems: Theory, Practice, Policy (RFF Press, 2010).
[14] M. Crotty, " Interview with Rainer Aringhoff," Beyond Zero Emissions, 14 May 09.