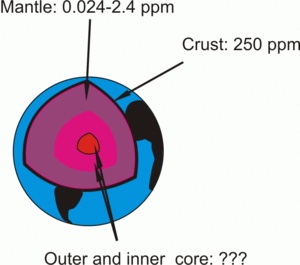
 |
| Fig. 1: Uranium concentrations in Earth's layers. |
Uranium oxide was first used in the first century b.c. for making glaze. First mention of uranium-containing mineral pitchblende came from the Saxon Mountains in 1565. Later reports of pitchblende came from 1727 (Joachimsthal) and 1763 (Schwarzwald). [1] In the beginning of 19th century, uranium was a co-product (and even waste!) of mining industry in Bohemia and England. First purposeful mining of radioactive ores started at Jachymov silver deposit in Czechia. It was radium from Jachymov that eventually killed Marie Curie in 1934. Uranium ores were mostly mined for radium before the WWII. Radium was required for making luminescent paint used in watches and instruments, and for medical purposes.
The situation changed drastically after the discovery of nuclear fission by Hahn and Strassman. There came an understanding that the discovery could lead to construction of "extremely powerful bombs of a new type" (letter of Albert Einstein to President Franklin Roosevelt). Einstein also mentioned in his (later regretted) letter that "The United States has only very poor ores of uranium in moderate quantities. There is some good ore in Canada and the former Czechoslovakia, while the most important source of uranium is Belgian Congo." The Manhattan project was to follow shortly after the letter. United States first satisfied its needs for uranium in the Belgian Congo. Then the ore was supplied by Eldorado Mining and Refining Limited Company from the U.S. southwest and Canada. Other countries were vigorously searching for radioactive ores as well. In USSR the infamous minister of Internal Affairs Lavrentiy Pavlochich Beria was appointed as the Head of Atomic Industry. Most of the Soviet geologists were exempt from going to war. Instead they were sent to the east in lengthy expeditions in the search for uranium (and other) ores.
Most of our knowledge about Earth's interior comes from geophysical (i.e. indirect) data. Rocks, which we can lay our hands on, are limited to crust and upper mantle origin. Obviously, the best studied rocks come from Earth's crust. Interestingly, the deepest well ever drilled measured 12290 meters (Kola Superdeep Borehole, Kola Peninsula). Our information about the mantle is limited to xenoliths (mantle inclusions, dragged to the upper crust by kimberlitic magmas), pieces of upper mantle protruded (squeezed) to surface, and hot spot magmatism.
The uranium concentration in Earth's crust was calculated by Vinogradov to be 2,5*10-4% (250 ppm). [2] There have been several studies [3-5] in which uranium concentrations of some mantle rocks were measured to be in the range of 0.024-2.4 ppm.
Direct measurements of uranium concentrations in Earth's core are not possible with current level of technology.
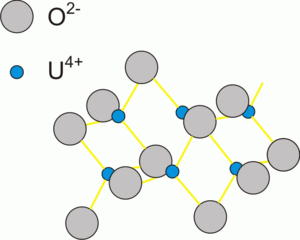 |
| Fig. 2: Uraninite atomic structure. Note, that this figure does not represent a unit cell of uraninite. |
An online mineralogy database [6] lists 249 uranium bearing minerals (this database is a compilation of several published books. By far, the most abundant of them is uraninite, a uranium dioxide UO2. Uraninite possesses a cubic crystal system, which implies equal linear dimensions (a = b = c = 5.4682 angstroms) and angles (α = β = γ = 90) of the unit cell. Each uranium atom is surrounded by 8 atoms of oxygen, which are in cubic coordination (form a cube around U). The unit cell contains 4 uranium and 8 oxygen atoms. Uraninite occurs both as well-deformed octahedral and cubic crystals and as grape-like" rounded forms, resembling malachite. It is dense (specific gravity = 10.6-10.9), diamagnetic, medium hard (5-6 on the Mohs scale). Uraninite was (officially) discovered in 1772.
A few factors are important in order for uranium to accumulate in one place and form a deposit. First of all, there must be a source rock. It does not have to possess anomalous concentrations of uranium, just some amounts of it. Second, a geochemical transporting agent is required. For example, hydrothermal veins are excellent in carrying different elements away from their source. Lastly, there needs to be a geochemical barrier. The latter corresponds to physical and chemical conditions, under which the carrier cannot hold a particular element. Pressure, temperature, and pH are some of such barriers. Possibility of having all factors play in a positive way greatly depends on the geodynamic situation.
Some possible geodynamic scenarios and associated uranium deposit types are shown on Fig. 4. Let me list the major parts of Earth's crust, and some possible geodynamic situations. Earth's outermost layer is lithosphere. It is broken into several rigid lithospheric plates which possess an ability to slide over astenosphere (partly melted layer of the mantle). Lithospheric plates are composed of both continental and oceanic lithosphere. Oceanic lithosphere is heavy, relatively thin (~10km), and possesses simple structure. The top most layer is deep water silisic sediments (also known as cherts). They are underlaid by basaltic pillow lavas, which owe their look to rapid cooling underwater. Next layer is sheeted dyke complex. Below them lay gabbros, followed by serpentinized peridotites. Continental lithosphere is much thicker - up to 200 km. It includes two major elements - cratons and fold-and-thrust belts. Cratons are ancient continental plates. Examples: Laurentia is an ancient craton, which is now a part of North American plate. Baltica and Siberian cratons are now incorporated into Eurasian plate. Any craton can be divided into two structural elements: crystalline basement and sedimentary cover. Areas, where basement rocks are exposed are called shields (e.g. Canadian shield, Baltic shield, Anabar shield, etc.). Fold-and-thrust belts correspond to ancient oceans that once were separating cratons. There are modern fold-and-thrust belts (e.g. Alp belt between African and Eurasian plates) and ancient ones (like Central-Asiatic belt in Eurasia).
There are three ways in which plates can interact with each other: convergence, divergence, and lateral movement. If a heavy oceanic plate converges with light continental lithosphere, it starts subducting under the latter, forming a chain of volcanoes (for example, the Kamchatka peninsula). Oceanic plate can also subduct under another oceanic plate, forming an island arc (e.g. Aleutian Islands). If two continental plates collide, neither of them sinks under the other. The excess energy results in orogeny (e.g. Himalayan Mountains, whose uprising resulted from India colliding with Eurasia). Divergence of plates away from each other results in creation of new oceanic crust. Shallow parts of it adjacent to the continent are called shelf.
Now we are ready to associate deposit types with geodynamic settings (most of the following information in this chapter was taken from [7]).
There are deposits associated with Precambrian shields (a shield is an exposed basement, so it is reasonable to assume that parts of basement, hidden under sedimentary cover, can also host uranium deposits). One place to look is an unconformity plane between crystalline rocks and overlying sedimentary rocks. Some of the well known unconformity related deposits are Cigar Lake (Canada) and Jabiluka (Australia). Another shield-related deposit type is quartz-pebble conglomerates. Archean granites were the source for conglomerates. Early Proterozoic rifting resulted in a system of narrow intracratonic basins where sediments were accumulating. Rivers and gravity were the carrying agents. Witwatersrand (South Africa) is an example of this deposit type.
Intrusive (plutonic) rocks are the ones that have crystallized from molten magma below Earth's surface. They vary in size, shape, and composition. Deposits associated with intrusive rocks are generally low-grade (20-500ppm), but may contain substantial resources (> 105 tonnes of U). [7] There are five known types of plutonic rocks that are hosts for uranium mineralization. They are: alaskite granites (e.g. Rossing in Namibia), monzonite granites (Bingham Canyon, Utah), syenites (Kvanefjeld, Greenland), carbonatites (Phalaborwa in South Africa), and pegmatites (Bancroft area in Ontario, Canada).
In addition, large granite bodies often have systems of hydrothermal veins. Ore grades in the veins are usually higher than those in parental plutonic bodies. Aforementioned Jachimov is a notable example of vein-related deposits.
Volcanic rocks are generated by magma that made its way to Earth's surface. Uranium deposits are often associated with calderas - large, bowl-shaped volcanic depressions, formed by the collapse of a volcano. Uranium mineralization occurs in post-eruption times and is connected to hydrothermal activities (e.g. fumaroles). Streltsovskoe in Russia is an example of caldera-related deposit.
Soils, porous sedimentary rocks, and karst caverns can be hosts for uranium. Uranium deposits in calcrete (calcium and magnesium carbonates) are the largest of surficial deposits. They form in regions where uranium-rich granites were deeply weathered in a semi-arid to arid climate. Once again, granites are the source rock, while water streams are the carrying agent. Yeelirrie and Lake Maitland in Western Australia are examples of surficial deposits.
Phosphorites are chemical and biological sedimentary rocks, which contain high amounts of phosphates. Source for biological phosphorites are bird droppings (a.k.a. guano). Chemical phosphorites are generated during erosion of rocks, containing high amounts of apatite. The uranium mineralization is substituted for calcium in cryptocrystalline flour-carbonate apatite grains. Phosphorite deposits contain large uranium resources. Unfortunately, they are very low grade (25-150 ppm).
Earth is losing heat. The total power dissipated from the Earth has been measured by thermal techniques to be 44.2 +/- 1.0 x 1012 watts. [8] Lord Kelvin calculated in 1862 that it would take Earth (with initial temperature = 3900 C) 20-400 million years in order to cool to its present surface temperature gradient. [9] We know, however, that Earth's age equals to 4.6 billion years [10]. This implies the necessity of a reheating mechanism. Heat, resulting from radioactive decay is a probable solution. An international group of scientists [11] has estimated the amount of heat introduced by decay of U238 and Th232. When those isotopes decay, they produce daughter nucleus, an electron, and an antineutrino. [11] measured the flux of antineutrinos at the Kamioka mine (Japan). They came to an estimate, that decay of the aforementioned isotopes could be responsible for 16 x 1012 of heat. This corresponds to one third of the total heat flux.
Before radioactive methods of dating were discovered, geologists were limited to using relative ages instead of absolute ones. Radioactivity allows us to possess a deeper knowledge about Earth's history. The idea behind geochronology is as follows. Several minerals (notably zircons) can incorporate some amounts of radioactive isotopes (e.g. U238) during or shortly after crystallization time. After cooling below a certain temperature, the isotopic system becomes closed to isotopes - it no longer (ideally) can accept or lose the particular isotope or its daughters. At this point the isotopic clock is set. Any future changes in lead and uranium concentrations are due to radioactive decay. By calculating daughter-to-parent isotope concentrations ratios scientists are able to determine the age of uranium-bearing mineral. More information on isotopic geochronology may be found in appropriate textbooks. [12]
© 2009 Slava Powerman. The author grants permission to copy, distribute and display this work in unaltered form, with attribution to the author, for noncommercial purposes only. All other rights, including commercial rights, are reserved to the author.
[1] F. J. Dahlkamp, "Uranium Ore Deposits" (Springer, 1993).
[2] A.P. Vinogradov, "Regularities in Distributions of Chemical Elements inside Earth's Crust" in Selected Papers: Problems in Geochemistry and Cosmochemistry (in Russian) (Nauka, Moscow, 1988)
[3] F. Aumento and R. D. Hyndman, "Uranium Content of the Oceanic Upper Mantle," Earth Planet. Sci. Lett. 12, 373 (1971).
[4] E. L. Haines and R.E. Zartman, "Uranium Concentrations and Distribution in Six Peridotite Inclusions of Probable Mantle Origin," Earth Planet. Sci. Lett. 20, 45 (1973).
[5] M.G. Seitz and S.R. Hart, "Uranium and Boron Distributions in some Oceanic Ultramafic Rocks," Earth Planet. Sci. Lett. 21, 97 (1973).
[7] World Distribution of Uranium Deposits (UDEPO) with Uranium Deposit Classification , (Intl. Atomic Energy Agency, 2009).
[8] H. N. Pollack, S. J. Hurter and J.R. Johnson, "Heat Flow from the Earth's Interior: Analysis of the Global Data Set," Rev. Geophys. 31, 267 (1993).
[9] J. D. Burchfield, Lord Kelvin and the Age of the Earth" (U. Chicago Press, 1990), p. 43.
[10] G. B. Dalrymple, "The Age of the Earth in the Twentieth Century: a Problem (Mostly) Solved." Special Publications, Geol. Soc. of London 190, 205 (2001).
[11] T. Araki et al., "Experimental Investigation of Geologically Produced Antineutrinos with KamLAND," Nature 436, 499 (2005).
[12] A. P. Dickin, Radiogenic Isotope Geology (Cambridge University Press, 1995).